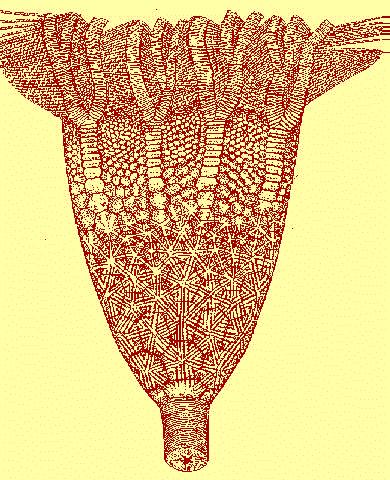
 a)
a) b)
b)|
|
Petr KÁCHA - Václav PETR
Abstract: A brief review of camouflage and mimicry in fossils is given. It is suggested that the great majority of palaeontological articles have referred to camouflage and mimicry only as in relation to discoveries of colours and colour patterns. The present authors stress the importance of morphology for the study of these phenomena in the fossil record and interesting possibilities of camouflage and mimicry (esp. in trilobites) are considered herein. Finally, both the structuralists´ as well as darwinists´ explanations of camouflage and mimicry are thouroughly discussed, discarded and a true holistic approach is proposed and defined herein.
"At the very least, as John Collier put it in a personal communication, "the separation of science from myth obscures the mythical character of science, and gives [it] more weight than it deserves."
Introduction
Camouflage and mimicry have long proved interesting and fascinating.
Every observer probably knows something of "masking" in the living world
because for every animal one easily discovers there are several scarcely
distinguishable or almost "invisible". Such "protective strategies" are
very common among animals. They are even overwhelming in the living world.
Without doubt, the same situation must be observable in the fossil record.
However, any observation or idea is interesting, but it is not really
a contribution to scientific knowledge until it is published for others
to read. But looking at the literature we come to a mysterious conclusion:
it seems that palaeontologists did not publish many articles on such common
phenomena under consideration herein. Why?
And what about zoologists? How far back in history we must go for the
first written observation of the phenomena discussed in our present paper?
Of course, the situation in zoology is different.
The writings of Greek philosophers contain material which is clearly
scientific showing that the Greeks made great strides in beginning to understand
the structure and function of the living world. In search for camouflage
and mimicry (these terms themselves are of recent coinage) we come to the
turning point in the history of Greek science, to a point of extensive
empirical enquiries and the last great systems of the world as a whole.
And we find that camouflage through active colour changes (described thoroughly
in the classical book, Parker 1948) in the octopus and chameleon was already
described by Aristotle (384-322 B.C.), founder of the Lyceum, who mentioned
it in his treatise "Historia Animalium". His observations became an important
part of scientific literature on the subject. References to camouflage
through colour changes in animals were made by Pliny, i.e. Gaius Plinius
Secundus (A. D. 23/24-79), in his "Naturalis Historia", too. As a recognized
distinct field in zoology, the interest in the phenomena of camouflage
and mimicry dates from the 19th century, especially thanks to the early
darwinists (see below). But what about the references to camouflage and
mimicry in fossils?
Although ancient invertebrate shells, skeletons and carapaces displaying
original colour patterns are among the rarest fossils, we can find a relatively
considerable number of articles on this subject (see especially the summary
and bibliography in Kobluk and Mapes 1989). Because of some special forms
of some preserved colour patterns, possible presence of the phenomena of
camouflage, warning coloration and mimicry in fossil marine organisms has
been described by several previous authors. For example, Cowen, Gertman
and Wiggins (1973), Kobluk and Hall (1976), Kobluk and Mapes (1989), and
Boucot (1981, 1990) have reported colour, colour patterns, including the
so-called countershading patterns (for the purpose to counteract the differential
illumination) and the so-called disruptive marginal patterns (which break
up a sharply defined outline) of various fossil shells, skeletons and carapaces
which may have served probably for offensive or defensive camouflage, partly
for species or sex recognition. Gottfried (1989) has observed cryptic and
the so-called disruptive coloration (colour markings which break up the
shape of the body) in a single specimen of Elonichthys sp. (Upper Palaeozoic
actinopterygian fish from Kansas), etc.
In general, the great majority of articles have referred to camouflage
and mimicry in fossils only as in relation to discoveries of colours and
colour patterns. On the other hand, many of the works on fossil colours
and colour patterns did not refer to camouflage and mimicry at all. The
oldest known fossil with preserved colour pattern (fan-like array of stripes
on the pygidium) is the Middle Cambrian trilobite species Anomocare vittata
(Raymond 1922). For illustration, several other patterned trilobites have
been described by previous authors (except Raymond 1922, see e.g., Williams
1930, Wells 1942, Teichert 1944, Garretson 1953, Harrington 1959, Hessler
1965, Esker 1968 or Babcock 1982). For an interesting bibliography on colour
patterns in Palaeozoic marine invertebrates see Kobluk and Mapes (1989).
The latter includes also the article on colour patterns of some Barrandian
(Lower Palaeozoic) fossils (Køí and Luke 1974), however, do not include
the works on colour patterns of some fossil invertebrates from the Barrandian
area which are in preparation (a larger work of Vojtìch Turek on colour
patterns in cephalopods, and two reports of Radvan Horný on colour patterns
discovered in platyceratids and cyrtonellids; both authors are members
of the Department of Palaeontology, National Museum).
Well-known are findings of colour and colour patterns on wings of fossil
insects (for summary see Boucot 1990, and Shear and Kukalová-Peck 1990)
and their possible function as warning or mimetic coloration has been discussed.
Also colour patterns on the insect´s abdomen are known in the fossil record
(see e.g., Becker 1965), colour patterns on the carapax of a turtle (Sullivan
et al. 1988), chromatophores in fossil anurans, colour patterns on feathers
of fossil birds, etc. (for bibliography see Boucot 1990). Some of these
works also discuss the possible function of colour patterns in camouflage,
warning coloration or mimicry.
Therefore, it is obvious again that to date, most of the work on fossil
camouflage and mimicry has been concentrated on finding colours or colour
patterns in the fossil record. Paradoxically, other characteristics of
camouflage and mimicry (including those which are the best observable in
the fossil record, i.e. morphological ones) do not play an important role
in the research of fossils.
In non-zoological literature, less weight has been put on other characteristics
of the discussed phenomena and almost no palaeontologists were able to
see them in their full complexity, i.e. as a result of the interplay between
colours, colour patterns, shape of the body, behaviour and the surrounding
environment, both "physicochemical" and biological. This is the reason
why palaeontologists did not publish many articles on the phenomena under
consideration. Of course, among zoologists it is well-known that not only
the colour pattern but a combination of it and shape provides the animals
with most elaborate and highly effective disguises. The zoologists (e.g.
Cott 1940, Wickler 1968, Robinson 1969, Heráò 1976 or Owen 1980) have also
pointed to the importance of animal behaviour associated with the camouflage
phenomenon because the latter works only under special conditions, i.e.
in relation to the appropriate environmental background and the appropriate
"positioning" of the camouflaged animal in that background.
Of course, it seems questionable whether camouflage and mimicry in
such a many-folded sense have ever been recognized in the fossil record.
But while it is generally accepted that there are many examples of similarities
in the fossil record resulting from close relationship (i.e. because of
common ancestry), there has been less enthusiasm for the notion that two
similar species at the same place and time are related as a "model" and
its "mimic" or that they were camouflaged in the same manner. For example,
Jan Bergström (1973, p. 43) correctly stated that "convergence-like similarities
have been commonly taken to indicate a close relationship but the development
of the same morphological characteristics along different phylogenetic
lineages may be due to a similar mode of life". Unfortunately, the "mode
of life" is frequently related only to "abiological" environment. Palaeontologists
typically discuss parallelism (i.e., development of similar characters
in different lineages of common ancestry) and convergence (i.e., homeomorphism
or similarity resulting from the same mode of life in unrelated phylogenetic
lineages) but almost no palaeontologist is able to suppose that there are
other possibilities. The purpose of the present article is to point out
that in the fossil record many similarities between species may have resulted
from a similar type of camouflage or mimicry.
In order to search for camouflage and mimicry in the fossil record,
palaeontologists must study the external morphology of fossil shells, skeletons
and carapaces and so take into account that some features of the external
morphology can be of no systematic value (e.g. the granulation on the exoskeletons
of trilobites discussed below). Such "taxonomically unuseful characteristics"
are of great importance for reconstructions of life habits and - of the
camouflage as well. Among trilobites, for instance, it seems very likely
that well-camouflaged polymorphic species do occur in the fossil record
(not only in recent arthropods), and that several external morphological
similarities (e.g. between asaphids, scutelluids and lichids) do not result
simply from an unspecified, in many cases poorly abstract and hypothetical
"similar mode of life", but also from the same type of camouflage or mimicry.
Even though many aspects of camouflage and mimicry in fossils have
been never thoroughly investigated, enough is known of living animals to
provide comparison with fossils. Many questions still have no answers.
Therefore, it seems important for the present authors to discuss these
phenomena as thoroughly as possible (including at least a brief summary
of zoological literature), so that other palaeontologists can use the informations
given below to advance our knowledge in this very interesting but still
almost intact field of palaeobiology. The presented first part of our study
is for those interested in general aspects of camouflage and mimicry, including
discussion on consequences for the evolutionary theory. The second part,
which is in preparation, will be devoted to granulation (as camouflage)
on trilobite carapaces (material from Lower Palaeozoic of the Barrandian
area).
Recent camouflage and mimicry
Camouflage or crypsis is one of nature´s most common "escape mechanisms".
Typically, the various colours and colour patterns allow organisms to blend
with their background (the so-called cryptic coloration). This "invisibility"
is observable everywhere. In many cases the strategy of camouflage is directed
only to a limited number of predators. For example, the stripes of a zebra
(although seemingly conspicuous for us in the zoo) represent a type of
effective camouflage in natural conditions. Surprisingly, after experienced
hunters and travellers (see Heráò 1976), the black and white stripes merge
well with the surrounding vegetation so that the zebra cannot be seen even
at a very short distance. Moreover, unlike humans, their main predators
- lions - are colour-blind.
After Needham (1974), a colour-specialist, the main, but not the only,
functions of integumental chromes are in the contrasting roles of crypsis
(camouflage) and semasis (advertisement). Camouflage (crypsis) is most
crucial for animals preyed upon but is common also in predators, both those
"which lie in wait for the prey and those which approach by stealth." Its
types are diverse, extending to countershading, shadow-elimination, matching
background colours, shape-disruption, including the phenomenon of "chromatropism",
or better, chromotaxis (the latter represents an interesting behaviour
of camouflaged animals in which they deliberately seek a matching background).
After Needham (1974), advertisement involves bright, conspicuous colours,
in large patches, with sharp boundaries and contrasts, and may be interpreted
by the "target" animal as either warning (aposematic) or welcoming (episematic).
Warning coloration may be very effective. After Carpenter (1921), using
the monkey Cercopithecus as a test-predator, about 84% of insect species
bearing warning colours were distasteful, while, in camouflaged ones, only
18%(!). The contrasting colour bands of wasps are the best known warning
pattern. Bright colours of male birds are the best-known examples of sexually
attracting (episematic, epigamic) displays. Needham (1974) has pointed
out that the latter "must often compromise with camouflage requirements
and in many cases the colours and bold patterns are hidden except during
the actual process of courtship." The episomatic subdivision (i.e. episematic
patterns attracting for purposes other than reproduction), is the least
familiar. Perhaps the best known are the lures of some deep sea fishes
(e.g., aggressive mimicry in anglerfishes) and certain trematodes (Needham
1974, p. 257). Possibly also the bright colours of sea anemones may have
this significance. An example of pseudaposematic (Batesian) mimicry may
be that of palatable insects (cockroaches) of unpalatable leaf beetles
and lady-bird beetles. (The latter examples from Needham´s work are given
only to illustrate the terminology which is in various authors different;
the term mimicry will be discussed below and only the simplest terminology
will be applied herein).
In the marine environment, many forms of camouflage and mimicry exist
and some have been described in the literature (see e.g., Fricke 1975).
However, despite the great interest in colour patterns in the sea, their
function is still poorly understood. Possibly better known (especially
in reef fishes) are brilliant colour patterns which serve hardly as a means
of camouflage but of communication. The "visual cues" (colour patterns
of the reef fishes) are invaluable because other type of communication
would be almost impossible in the clear-water high-energy environment on
the reef (e.g. chemical cues are rapidly dispersed in such an environment).
For example, according to observations of numerous eye-colour patterns
in many reef fishes of the tropical western Atlantic less than 25% of the
species have camouflaged eyes (i.e. patterns disrupting the outline of
the eye for the purpose of concealment). On the other hand (see Tresher
1978), the most frequently encountered form of eye ornamentation is outlining
(i.e., a way to make an eye conspicuous). Moreover, because reef fishes
are best able to see blue and yellow, their pupil is often dark blue, while
their iris generally bright yellow.
Many animals perfectly imitate plants or inanimate objects (but also
plants may use such a technique), often with the aid of special behaviour,
e.g. a horned frog, a fish or a bush-cricket looking all like leaves, a
tropical mantid looking like a flower, etc. (see e.g. the summary in Owen
1980). Other interesting practice of "invisibility" is the camouflage of
a nightjar with plumage like bark and withering leaves, or the camouflage
of many bark-like bark-resting insects that spend much of their lives exposed
on tree trunks. Well-known are camouflaged lepidopterous insects. Especially
famous is the case of the so-called "industrial melanism" in peppered moth
Biston betularia (which represents also a kind of "adaptive polymorphism"
- see below, as well as of the above mentioned chromotaxis) described in
the classical article of Kettlewell (1959). Camouflaged nonlepidopterous
bark-resting insects are less well known (e.g. Silberglied and Aiello 1980).
The term mimicry, on the other hand, is generally used in connection
with animals that (especially visually) imitate other animals or their
parts (see Rothschild 1967) and, moreover, the "model" animal must be already
protected by warning coloration (it was the co-author of the theory of
natural selection, Alfred Russell Wallace, who has first propounded the
theory of warning coloration). Although Rothschild stressed in her article
that this is a somewhat confusing restriction of the term, for specialists
in this field it is still the situation of present days (Owen 1980).
The term itself has been introduced into biology by Kirby and Spence
(1817) but not in its present state of understanding. The true discoverer
of the phenomenon of mimicry was the British naturalists Henry Walter Bates
(*1825+1892). Bates, while voyaging along the Amazon Valley, found a perfect
visual resemblance of some butterflies to their highly protected heliconid
"models". Bates´s famous pioneering report - "Contribution to an Insect
Fauna of the Amazon Valley" - was read before the Linnean Society in June
21, 1861. Therefore, the so-called disguises of "sheeps in wolf´s clothing"
are named Batesian mimicry. The second main subdivision (Müllerian mimicry,
in which both the mim and model are unpalatable) is named after the German
zoologist Fritz Müller (*1822+1897).
George St. Mivart, a very popular Darwin´s opponent, has summarized
Bates´s discovery and Wallace´s theory:
"Let us take the cases of mimicry amongst Lepidoptera and other insects.
Of this subject Mr. Wallace has given a most interesting and complete account,
showing in how many and strange instances this superficial resemblance
by one creature to some other quite distinct creature acts as a safeguard
to the first. One or two instances must here suffice. In South America
there is a family of butterflies, termed Heliconidae, which is very conspicuously
coloured and slow in flight, and yet the individuals abound in prodigious
numbers, and take no precautions to conceal themselves, even when at rest
during the night. Mr. Bates (the author of the very popular book "The Naturalist
on the River Amazons," and the discoverer of "Mimicry") found that these
conspicuous butterflies had a very strong and disagreeable odour; so much
so that any one handling them and squeezing them, as a collector must do,
has his fingers stained and so infected by the smell as to require time
and much trouble to remove it.
It is suggested that this unpleasant quality is the cause of the abundance
of the Heliconidae; Mr. Bates and other observers reporting that they have
never seen them attacked by the birds, reptiles, or insects which prey
upon other Lepidoptera. Now it is a curious fact that very different South
American butterflies put on, as it were, the exact dress of these offensive
beauties and mimic them even in their mode of flight." (Mivart 1871, p.
33-34).
The present state of understanding is not very different. We can define
the visual mimicry (the most common and possibly the only type of mimicry
detectable in the fossil record) exactly as follows:
1) there must be an animal, so-called "mimic" which resembles another
species or "model" (also animal) and (from this resemblance) gains some
sort of advantage for itself,
2) there must be a model (typically more abundant than the mimic, esp.
in the Batesian mimicry) in the same place and time (the latter restriction
seems to be very important for zoologists as well as for palaeontologists)
which leads a "warning way of life" and which is imitated by the mimic,
and
3) there must be an "operator" (audience), generally a predator which
mistakes the mimic for the model (see e.g., Owen 1980).
Batesian and Müllerian mimicry studied extensively among arthropods
and some other invertebrates (see e.g., Poulton 1890, Brower and Brower
1962, Wickler 1968, Vane-Wright 1976 or Owen 1980) are relatively rare
(but only relatively!) in vertebrates (see e.g., Cott 1940, Heráò 1976,
Greene and McDiarmid 1981, Diamond 1994).
A special case of mimicry (unfortunately, some authors have excluded
it from the division of true mimicry), well-known in butterflies of the
family Lycaenidae, so-called diverting "false head", seems to be also very
effective form of disguise because a considerable number of predators is
known to perform their initial strike at the prey´s head (see e.g., Swynnerton
1926, Carpenter 1941, Curio 1965).
There are also other kinds of camouflage and mimicry and some of them
will be discussed below. For understanding of the further discussions it
is crucial to remember again and again that camouflage and mimicry do not
represent only a function of colour and colour patterns!
Because colours and colour patterns in fossils are rather a preservational
curiosity (at least for collectors), no doubt that in the articles written
by Lamont (esp. 1967, 1969) we can find the most important attempts to
count camouflage and mimicry in fossils. It is because the popular Scottish
palaeontologist Archie Lamont has given a first real confirmation of the
correctness of the way of explaining the function of camouflage and mimicry
in fossils from the viewpoint of morphological characteristics of ancient
animals´ skeletons. In other words, he has proved that camouflage and mimicry
are not curiosities in the fossil record. His provocative conclusions include
many aspects of the phenomena in many groups of Lower Palaeozoic marine
animals, e.g. aggressive mimicry in the eurypterid Stylonurus, mimicry
of "gaping mouth" threat in the tails of recent and fossil fish-shaped
vertebrates, several types of imitations of algae in fossils, a considerable
number of camouflaged trilobites (e.g., granulation imitating grit), several
types of camouflage and mimicry in brachiopods, graptolite colonies imitating
a brittlestar, etc.
A possibility of mimicry among trilobites has been also reported by
the American palaeontologist and macroevolutionist, Niles Eldredge. He
has mentioned calmoniids of the genera Schizostylus and Probolops and pointed
to the striking similarity of their heads, their common occurrence in the
same beds and to the rarity of the latter genus. Eldredge (1980, p. 50)
has concluded that Probolops is a (probably Batesian) mimic of Schizostylus.
From the same point of view, the last and most recent description of
a mimicry in the fossil record (and the first one among dinosaurs!) is
that of the Australian vertebrate palaeontologist, Tony Thulborn. He has
found that there is a very limited flexibility in Late Cretaceous ankylosaurid
tails, the clubs of which are usually regarded as a weapon to deter predators.
His conclusion is that this is a kind of mimicry of "false head" that may
have diverted predator (which had tendency to bite the neck or head) from
the true head. The ankylosaurid tail with its club may have imitated a
generalized form of neck and head of some other ornithischian dinosaurs.
According to Thulborn´s model, the carnosaur was "lured within reach of
the tail club" and "the tail club would strike at the aggressor´s head,
rather than its feet, thereby achieving the greatest deterrent effect"
(Thulborn 1994). Such an effective advertisement may have been possibly
multiplied by the "invisibility" of the true head. The author points to
a similar situation in other ankylosaurids, nodosaurids and stegosaurs
which have "a shallow skull that merges insensibly into a short neck."
It is possible that some or very many examples of species diversity
in the fossil record and even the so-called "ecophenotypic" variations
in a single fossil species have resulted from camouflage. We argue that
camouflage-related variability (and similarity, too) is common in recent
seas as well as on the land. However, palaeontologists have traditionally
searched only for the distinction of "environmental" from "genetic" variations
(see Johnson 1981) but the ancient animals did not live in a sort of abiological
laboratory environment.
As a preliminary result of our study of Lower Palaeozoic trilobites
(supposed to be a typical prey of large predators in Palaeozoic seas) is
that the size and density of granulation preserved on the external part
of trilobite carapaces may have been (in many cases!) a function of sediment
particle size. In other words, specimens of trilobites coming from fine-grained
sediments bear dense, soft granulation on their carapaces (in extremely
fine-grained sediments the carapax may be smooth), while those from coarse-grained
ones are provided with large granules scattered on the outer surface of
the exoskeletons. Such an interesting correlation has been previously recognized
by Chlupáè (1977) in some phacopid trilobites from the Barrandian Lower
Devonian, especially in Boeckops boecki (Hawle et Corda), Reedops bronni
(Barrande), and R. cephalotes (Hawle et Corda) (personal communication).
Similar relationship between the substrate particle size and the type of
granulation on the trilobite carapax we have found in the common dalmanitid
Dalmanitina socialis (Barrande) from the Bohemian Middle Ordovician. There
are good reasons (supported by shared knowledge and kind personal communications
of Prof. I. Chlupáè, Charles University) for the believe that the above
mentioned examples represent a relatively frequent phenomenon which points
to the presence of a specific and very common type of camouflage in trilobites,
regardless conclusions of previous authors about the taxonomic or non-taxonomic
value of the trilobite granulation.
Kácha and ariè (1991, 1994) stressed on some examples that the form
and even presence of trilobite granulation is dependent on the stage of
ontogeny. It is important because several previous works on Barrandian
trilobites has overemphasized the role of granulation for taxonomical purposes
and many polymorphic species received two, three or even more specific
names thanks to slightly different granulation. Moreover, although the
changes in granulation throughout the trilobite ontogenies are well known
in the literature (e.g. Chatterton, Siveter, Edgecombe and Hunt 1990),
they have been never related to camouflage. In some recent articles they
are even attributed to characteristics invaluable for phylogenetic analyses
(see e. g., Edgecombe, Speyer and Chatterton 1988). But the simple fact
that the more ontogenetically younger the individual the coarser and more
sparse the granules on its exoskeleton indicates relatively stable diameter
of the granules, points to a possible connection of the granulation with
the grain size of substrate (rather than to any phylogenetical conclusion).
It is important to stress that some external features of the trilobite
exoskeleton have been previously also related to the grain size of the
inhabited substrate. A constructional morphologist Helmut Schmalfuss has
already discovered that the "cuticular terraces" on the carapaces of some
trilobites (as well as those of some recent decapods) may have functioned
as to provide frictional resistance in interaction with the grains of the
substrate (Schmalfuss 1978a-c, 1981). The most interesting discovery is
that the number of these terraces per surface unit remains constant during
ontogeny (the grain-size of the inhabited substrate remains constant during
the animal´s ontogeny, too). We argue that such a close relation to the
grain-size of the substrate is also visible in the granulation: the number
of granules per surface unit remains relatively constant during the development
of many trilobites. Of course, this latter observation cannot be linked
with a possibility of burrowing life habits in these trilobites but rather
with camouflage purposes!
A possibility of the mimicry of "false head" in illaenid trilobites
is self-evident but the modern interpretation of illaenid morphology (cephalon
extremely similar to pygidium) is a different one. Westrop (1983) has interpreted
the life habits of the Ordovician illaenine trilobite Bumastoides on the
basis of its smooth skeletal surface, strongly convex cephalon and the
nature of the thoracic articulation as an "infaunal trilobite that was
poorly suited to epifaunal crawling" and proposed for it a "sedentary,
infaunal suspension feeding existence". The latter author has noted that
this mode of life would be applicable to many other "illaenimorphs" and
that "convergence has led to the appearance of effaced, strongly convex
morphotype in a number of unrelated families, including the Illaenidae,
Asaphidae, Aulacopleuridae, Plethopeltidae and Scutelluidae". However,
we argue that a mimicry of "false head" may also represent a plausible
explanation - for illaenids as well as for almost all other trilobites
(the similarity of the cephalon and pygidium is almost universal among
trilobites). Unfortunately, as we have incomplete informations (some will
remain incomplete probably forever) we can´t know exactly whether this
type of mimicry has been accompanied with a special colour patterns, "eye
spots" or a special behaviour. Finally, in a somewhat similar way, species
bearing large telsons, may have used them as diverting structures.
There are possibilities of a common presence of many types of camouflage
and mimicry in trilobites and several ones have been discussed previously
by Lamont (1967, 1969). Unfortunately, his conclusions have been typically
ignored and never followed in the works of specialists. It is possibly
because palaeontologists like to work after the "fashion". Archie Lamont
wrote:
"The study of mimicry at Birmingham University was unpopular, and Miss
Stephenson (1946) had just left. Mimesis upset neat Darwinian lineages
and introduced ideas of mutual aid instead of crude natural selection."
(Lamont 1969, p. 88).
The inevitable question for the present authors is whether the "mechanism"
of diversification of the trilobite fauna is well-explained in the literature.
In the two following chapters we will see that the answer is very important
for our further considerations on a specific type of camouflage.
Recent macroevolutionary theory, sometimes called "hierarchy theory",
has originated from the famous concept of punctuated equilibria (the pioneering
article is that of Eldredge and Gould 1972) which has been formulated to
account for the incompatibility of the so-called "phyletic gradualism"
with the fossil record. The "punctuation" is consistent with rapid allopatric
speciation, while the "equilibrium" with long periods of stasis. The hierarchy
theory, at least in the American literature, has replaced in most cases
the former neo-Darwinian "modern synthesis" and may be summarized as follows:
"'Hierarchy theory' accepts the neo-Darwinian paradigm of within-population
variation, selection, and drift, but seeks to extend the list of evolutionary
entities beyond genes, organisms, and populations. Specifically, species,
monophyletic (higher) taxa, and ecosystems have come to be viewed as having
real existence, and are variously termed 'systems', 'entities', or even
'individuals'. The goal of hierarchy analysis is to elucidate the nature
of each kind of large-scale entity, and thus to determine their possible
role(s) in the evolutionary process." (Eldredge 1990).
Some orthodox neo-darwinists (i.e. representatives of the so-called
"modern synthesis") claim that this is a misleading and completely non-Darwinian
approach to the evolutionary theory, while other neo-darwinists (also "synthetitists")
claim that there is nothing new under the sun and that similar model was
previously proposed in his classical works by George Gaylord Simpson (e.g.,
Simpson 1944, 1949, 1953). There are many reasons for this curious situation
but possibly the most distinct is that in the Eldredge´s (seemingly holistic)
definition - no smooth extrapolation of microevolutionary processes (i.e.
processes within a population) can lead to macroevolutionary phenomena
(i.e. among species) and, especially, that there are very different views
of "natural selection" and its bearing on evolution.
The "punctuated equilibria" originated as an idea of Niles Eldredge
(see e.g. Eldredge 1971) who studied Lower Palaeozoic trilobites and observed
that species persisted for long periods of time without change. Therefore
(especially in Eldredge 1974, 1980) the role of natural selection in creating
new species has been deeply questioned. Eldredge and Gould (1972, see also
Gould and Eldredge 1977) pointed to the fact that species are real entities
not only in space but in time as well and proposed an allopatric model
of speciation which gained (after a decade) nearly total acceptance among
palaeontologists. From this point of view speciation is a fundamentally
accidental fragmentation of a once coherent population and natural selection
accounts only for a small (if any) part of this rapid change:
"There is as yet no proven, necessary relationship between natural
selection and speciation. Though selection invariably plays an important
role during speciation event, it has never been shown to be the effective
"cause" of speciation in the sense that the selective regime originates
and is sustained for the "purpose" of developing a new species." (Eldredge
1974).
"... natural selection per se does not work to create new species.
The pattern of change in so many examples in the fossil record is far more
a reflection of the origin and differential survival (selective extinction)
of species than the inexorable accumulation of minute changes within species
through the agency of natural selection." (Eldredge 1980, p. 51).
From this point of view in the "punctuated equilibrium" model the change
is not inevitable as it is in the orthodox selectionist model ("modern
synthesis") where the "feeling that evolutionary change is almost inevitable
is based upon the relentless logic of adaptation through natural selection:
somehow, one feels, it is inevitable that environments must change, and
organisms must modify their adaptations to keep pace, or face the grim
consequence of extinction." (Eldredge 1984).
Because the more heterogeneous the environment is, the greater the
possibilities of establishment of isolates and of speciation. In the sea,
it is far easier to envision habitat heterogeneity along "onshore" than
"offshore" areas. Therefore, for the evolution in environmentally monotonous
Palaeozoic epeiric seas the most important issue of the speciation model
of Eldredge and Gould (1972) is that proposed by Eldredge (1974). The latter
pointed to the possibility that monotonous offshore areas of the epeiric
seas "allowed little real opportunity for establishing genetic discontinuity
among populations of a species." (Eldredge 1974, p. 544).
Therefore, Niles Eldredge has argued that offshore areas (areas with
high diversity) have their source of new species in onshore areas (areas
with low diversity). In other words, the latter author really agreed that
more species are found in offshore areas (because of the greater degree
of "niche subdivision" arising from environmental stability) but argued
that the exploitation of these areas resulted probably from speciation
in onshore areas.
Eldredge´s theory, mounted, however, on the myth of competition between
"primitive" and "advanced" animal groups, is now well-known as "onshore-offshore
pattern" or "onshore-offshore faunal change" and may be ascribed to J.
J. Sepkoski, Jr. (see e.g., Sepkoski 1981, 1991, 1995, Sepkoski and Miller
1985, Sepkoski and Sheehan 1983).
The latter author proposed the "Archaic-Cambrian", "Palaeozoic" and
"Modern" "Great evolutionary faunas" from which each originated onshore
and displaced the earlier one to offshore areas during the course of time.
However, some specialists disagree with Sepkoski´s propositions. For example,
Webby (1992) points to the fact that Sepkoski believes that Ordovician
radiations are produced by a great expansion of the "Palaeozoic evolutionary
fauna" (e.g. Trilobita and Eocrinoidea are assigned to the so-called "Cambrian
fauna", while all other echinoderms, except Echinoidea and Holothuroidea
to the "Palaeozoic fauna") but that the highest levels of the productivity
of new higher taxa and community types are in the mid-outer shelf and slope
habitats, not onshore.
It is obvious that there is a problem with the so-called "incomplete
fossil record". This is a problem as old as the Darwinian theory of evolution
itself. In this case it is evident that, for example, during the Ordovician
the "Palaeozoic" fauna diversified onshore while the evidence in the fossil
record points to higher diversity offshore. But for neo-darwinists, both
orthodox and heterodox, this is possibly (traditionally) only a minor pitfall.
Even the macroevolutionists (heterodox neo-darwinists) argue, for example,
that:
"These discordancies (in onshore-offshore pattern between well-preserved
orders and well-preserved genera and poorly preserved orders in post-Palaeozoic
marine benthic invertebrates) suggest that the pattern of preferential
onshore origination (of post-Palaeozoic benthic invertebrates) is not an
artifact of preservation or collection and that the origin of higher taxa
cannot be regarded as a simple extrapolation of rates and patterns at lower
levels." (Jablonski and Bottjer 1991).
A much more serious problem, however, poses the question of genetic
variability within a species. In a recent textbook on biology there is
well-expressed a common argument of neutralists´ against the evolution
through natural selection: "These critics (i.e. neutralists) point out
that if natural selection is so important, then species surviving in changeable
environments should show more genetic variability, and those in stable
environments should show less. One might predict, for example, that species
in the chaotic intertidal zone would show more genetic variability than
species living on the stable floor of the deep sea. Both groups, however,
show equal genetic variability when tested." (Postlethwait and Hopson 1989)
So, the evidence conflicts with predictions of orthodox selectionists
for which the genetic variability must be greater in the intertidal (and
for which the origin of higher taxa is a "simple extrapolation of rates
and patterns at lower levels") as well as with those of modern "holistic"
macroevolutionists (accepting paradoxically "the neo-Darwinian paradigm
of within-population variation, selection, and drift", but seeking "to
extend the list of evolutionary entities beyond genes, organisms, and populations"
because "the origin of higher taxa cannot be regarded as a simple extrapolation
of rates and patterns at lower levels"). However, it seems to be evident
that, at least in some cases, the places of maximum species diversity in
particular groups tended to shift progressively offshore over time (Sepkoski
1991). Why?
So far, we have dealt only with a certain type of data interpretations
but the "patterns" may be explained in other, much more plausible ways.
Westrop, Tremblay and Landing (1995) studied the well-known declining importance
of trilobites from Cambrian to Ordovician and the possible "onshore-offshore
pattern" in trilobite evolution. They accepted that during the Cambrian
and Ordovician there is a general gradient of increasing trilobite species
diversity from nearshore (onshore) to offshore environments. However, they
simply argued that the places with maximum species diversity (i. e., offshore
environments) were really those in which trilobites (in situ!) attained
their maximum species diversity. According to new data from northern North
America, Westrop, Tremblay and Landing (1995) found that trilobite species
in nearshore (onshore) settings maintained a constant(!), low level diversity
between the Late Cambrian and Middle Ordovician (there is no evidence for
the predicted trilobite species-diversity decline in nearshore environments).
Therefore, the latter authors pointed out that there is no observable
replacement of "Cambrian fauna" (trilobite-rich palaeocommunities) by "Palaeozoic
fauna" (brachiopod-dominated) and "Modern fauna" (mollusc-dominated), that
the relative importance of trilobites declined rather through a "process
of dilution" as species of newly radiating groups (e.g., gastropods, articulate
brachiopods) joined them in their environments, and that the "apparent
offshore retreat of trilobite-dominated palaeocommunities (i.e. of the
"Cambrian fauna") is also at least partly a reflection of this dilution
process". Moreover, these authors stressed that the data from the District
of Mackenzie indicate little change in trilobite species diversity even
in Middle Ordovician offshore environments.
They concluded that: "... trilobite-dominated paleocommunities maintained
their integrity longer in the offshore, and this produced an overall pattern
of diachronous replacement. Similarly, progressive offshore replacements
of other community types in younger strata may also be dilution phenomena
related to species diversity gradients... There does not appear to have
been any active ecological displacement of one group by another." (Westrop,
Tremblay and Landing 1995).
Keeping in mind the model of trilobite diversification outlined above
and especially the exclusion of the hypothetical displacement of "primitive"
groups by "advanced" ones, we come to polymorphic trilobite species.
After the classic of "modern synthesis", Theodosius Dobzhansky, the
"adaptive polymorphism" is a widespread phenomenon in the living world.
He pointed out that "adaptively polymorphic populations should, in general,
be more efficient in the exploitation of ecological opportunities of an
environment than genetically uniform ones..." and that "A species will
be polymorphic if it contains a variety of genotypes each of which is superior
in adaptive value to the others in some habitats which occur regularly
in the territory occupied by this species" (Dobzhansky 1951).
The latter author argued that the so-called industrial melanism is
a kind of this phenomenon and that "becoming inconspicuous in normal surroundings,
or acquiring a resemblance to some object which is dangerous or distasteful
to natural enemies, are among the possible ways in which an organism may
become adapted to its environment".
There seems to be an interesting application of the latter words in
trilobites. It has been observed (e.g., Whittington 1966, Foote 1990) that
the trilobite suprageneric taxa are morphologically more distinct from
each other in the Ordovician than in the Cambrian. Hughes (1991) has argued
that there was a progressive change in species discreteness in trilobites
from the early Cambrian to late Ordovician and that also many other studies
provide quantitative evidence which suggests that the degree of "canalization"
in trilobite species increased through the Palaeozoic. The latter author
points out that results show that species belonging to "primitive" clades
show greater allometry than species belonging to "advanced" clades, that
the species from "primitive clades" often show striking changes throughout
adult growth, and that higher levels of ontogenetically invariant intrapopulational
variation also occur within trilobite species from "primitive clades".
We must ask again, however: Is it reasonable to see such a phenomenon
as a result of "primitive" and "advanced" characteristics? Certainly not.
Even Charles Darwin himself was well aware of the difficulties of the concept
of "higher" and "lower" and Ghiselin (1969, p. 70) has pointed to the fact
that Darwin made a memorandum in his copy of Robert Chambers´s book "Vestiges
of the Natural History of Creation," writing "Never use the word ´higher´
and ´lower´"! Darwinists, including those from the time of "modern synthesis",
all disagree with the words "primitive" and "advanced", "higher" and "lower"
etc. since the classical work of Williams (1966). The same opinion we can
find in works of all recent macroevolutionists - for possibly the best
analysis of the problem see Benton (1987). The syntheticist Michael Ghiselin
even wrote:
"The idea of absolute scale for ´highness´ or ´lowliness´ is an example
of essentialist metaphysics which is very common in our everyday thought,
and which has profound social implications." (Ghiselin 1969, p. 71).
Foote (1990) analyzed mathematically morphological diversification
of Cambrian and Ordovician trilobites and supported the opinion that "morphospace
became more discontinuously occupied from the Cambrian to the Ordovician".
He pointed out that the "world became a progressively more constrained
place for organisms" and that this increase in morphological clustering
might be interpreted to reflect a "transition to an adaptive landscape
marked by steeper peaks and deeper valleys."
On the contrary, the latter author has observed also a clear increase
in clustering intensity from the Cambrian to the Ordovician (i.e., the
morphological clusters are tighter in the Ordovician than in the Cambrian).
He concluded that these tighter clusters "might represent groups of species
with more finely partitioned niches". Moreover, Foote (1990) has expected
such results and suggested that the expansion of "morphospace" logically
accompanied the diversification.
Yes, it seems to be self-evident that the tighter clustering "may be
an expected consequence of any diversification", however, the validity
of this conclusion rests implicitly on the curious assumption that there
is an infinite number of "niches" in the "abiological" environment to which
the organisms "are adapted". Moreover, the diversification is "implied"
by adaptation which is "implied" by the action of natural selection. However,
the largest disparity of marine arthropods is observable in the fossil
record from Lower and Middle Cambrian (Gould 1989), i.e. from the time
in which the "niches" (from the orthodox view) were paradoxically extremely
undifferentiated. Therefore, we agree with Rosen (1978, p. 372) that "Although
natural selection theory fails to explain the origin of evolutionary novelties,
its greatest shortcoming in terms of evolutionary theory is that it fails
to explain evolutionary diversity."
However, we do not suggest that the interesting discoveries of Foote
(1990) and Hughes (1991) are erroneous. Quite the contrary. We greatly
appreciate their discoveries (not explanations) because they are very important
for our purpose to search for camouflage and mimicry in the fossil record
of trilobites.
The evidence that older or "primitive" trilobite species show a reduced
degree of developmental "canalization" compared to younger or "advanced"
ones meshes well with the hypothetical model of Kobluk and Mapes (1989)
in which the colour patterns did not function originally as camouflage
and warning displays. The latter authors studied Palaeozoic invertebrates
(about 180 Palaeozoic genera are known with colour patterns preserved!)
and pointed to distinctly increasing diversity of colour patterns in the
fossil record. They proposed three developmental phases in the evolution
of invertebrate colour patterns:
1. the origin of colour patterns during the Cambrian as simple incorporation
of metabolic by-products into the invertebrate skeletons,
2. use of these pigments for other purposes, e.g. light screening,
and
3. use of these pigments and colour patterns for camouflage, mimicry,
and warning display. Kobluk and Mapes (1989) argued that the colour patterns
in the earliest Palaeozoic may have developed prior to the evolution of
predators with "vision sufficiently sophisticated to see them" (i.e., colour
patterns).
Both the latter important discoveries (i.e., Foote 1990, and Hughes
1991 on the one hand, and Kobluk and Mapes 1989 on the other) are significant
for our conclusion that both are very closely interrelated due to predators´
views of prey in the Palaeozoic seas. It is self-evident. The unusually
high degrees of intraspecific and interspecific variations among trilobites
within the earliest Palaeozoic "primitive" groups ("weakly canalized growth
regulation" has been invoked to explain this variability by Hughes 1991)
may have represent the only way to reduce the likelihood of being captured
(and eaten) by a predator with extremely poorly sophisticated vision.
This explanation is theoretical and is based on the above mentioned
evidence in the fossil record and on what is believed to be the way in
which some predators search for their prey. Predators learn by experience
and consistently seek more of a species with which they have already had
success. Because of previous experience, predator forms an image of what
it is looking for and goes on searching for the same species.
Recent species with distinct morphologies are typically camouflaged
by colour patterns that match the background either by blending or by resemblance
to a specific structure. In some cases, however, the species use methodology
of "being different" (see e.g., Owen 1980). For example, in the recent
brittlestar Ophiopholis aculeata, there is an extraordinary variation in
colour, colour patterns and morphological characteristics. The predator
must examine hundreds of specimens before finding that two are alike. In
the case of predators with poorly sophisticated vision in the earliest
Palaeozoic seas, an important possibility to reduce their responsibility
to learn may have been (for the prey) paradoxically to look all alike (no
distinct species-specific morphologies developed) and being all different
(almost each individual is different from others in a given species). And
this forms a logical explanation of the trend discovered in trilobites,
and for which the changing composition of ecological-ethological types
of predators seems to be clearly responsible.
This question has been thoroughly discussed in Babcock (1993a). We can
only add some remarks.
The earliest predators were possibly large arthropods, unable to see
colours. Babcock and Robison (1989) examined the scars on Palaeozoic trilobites
and reported that those scars that are resulted from unsuccessful (sublethal)
predation (rather than from undefined causes) are significantly more frequently
found on the posterior and right side of the trilobites. In Lower and Middle
Cambrian trilobites these scars are frequently attributed to Anomalocaris.
Babcock (1993b) stressed that this "strong lateral asymmetry of predation
scars is most likely due to handedness in either the predators or the trilobites
or perhaps in both". Therefore, Babcock and Robison (1989) concluded that
handedness (or "lateralized behaviour") had developed in the Cambrian seas
- about 500 million years earlier than previously supposed (see also Babcock
1993 a,b).
Regarding the evidence of large arthropods preying on trilobites in
the Barrandian area, there are some possibilities in the Ordovician rocks
(cephalopods are important from the Silurian onward), e.g. in the Letná
Formation. After personal communication of Prof. I. Chlupáè (Institute
of Geology and Palaeontology, Charles University), "Nothozoe" barrandei
ChLUPÁÈ (see Chlupáè 1970) of the Letná Formation at Drabov represents
the only phyllocarid attaining a sufficient length (up to 40 cm) of a large
predator. But there is a considerably larger predator than "Nothozoe" at
the above mentioned locality. In personal collections of Prof. I. Chlupáè
there are several fragments of giant, smooth carapaces of a possible eurypterid.
Prof. I. Chlupáè (personal communication) supposes that the length of this
undescribed and poorly known creature was probably about 1 m.
Regarding Lower Palaeozoic cephalopods (in Barrandian they are important
in Silurian and Devonian), almost no inference may be drawn from the evidence
about their vision. Russel-Hunter (1968, p. 160) has pointed to the fact
that there are behavioural indications of colour vision of recent cephalopods
and that their eyes are certainly the dominant sense-organs in their behavioural
patterns. However, the situation with Nautilus (possible nearest neighbour
of Palaeozoic forms) is much more complicated. Wells (1962, p. 146-147)
wrote that Nautilus "... has large eyes of simple structure, with no lens
and no iris. There is a very small circular pupil. The eyes can move backwards
and forwards about a vertical axis, but the animals do not appear to recognize
objects by sight. They are, however, clearly very sensitive to changes
in light intensity, and in aquaria remain quiescent and withdrawn during
the day, emerging to swim about only when the light intensity fades towards
sundown. While they are attracted to lights and will follow an electric
torch about an aquarium, they tend to seek shade from more general illumination..."
and that "In the sea, as in aquaria, Nautilus seems to locate its food
by smell rather than by sight..." and that "they depend on chemotactile
sense rather than on sight to locate their food." The latter author and
Lane (1957) also pointed to old observations of Arthur Willey (reference
in Lane 1957, Wells 1962) who reported that "basket trap baited with cooked
crustaceans concealed under coconut fibre caught more Nautilus than similar
traps baited with freshly dead, clearly visible fish. The traps were set
in clear water at a depth of about one hundred metres." On the other hand,
we must stress that several different extinct groups of cephalopods are
known from the Lower Palaeozoic rocks and we know nothing about their real
capacity of vision. Ward (1982) has observed that Nautilus macromphalus
commonly gains nutrition eating molted exoskeletons (exuviae) of spiny
lobsters (only the hard cephalothorax is left untouched).
From the uppermost Silurian onward (in the Barrandian area as well
as in the world) there is a real possibility of predators with well-sophisticated
colour vision (i.e., true fishes). Such an occurrence meshes well with
both the observations of Kobluk and Mapes (1989) as well as with those
of Foote (1990) and Hughes (1991).
It is generally supposed that one measure of natural sciences is the
understanding of natural phenomena and of man´s place in nature. In the
search for this understanding naturalists have called upon gods, evolution,
archetypes, spirits, transcendentalism, lamarckism, functionalism, structuralism,
darwinism, holism, reductionism, vitalism, etc. But the search does not
constitute a steady progress of scientific advance or a continuous growth
of scientific knowledge: it is rather a matter of changing paradigms (Kuhn
1962, 1970). Because camouflage and mimicry represent phenomena the explanations
of which are extremely "paradigm-sensitive" (i.e., their existence may
be either denied, as by some structuralists, or explained through "holistic
downward causation", as by creationists and darwinists, see below), we
must at least touch these related topics in the following chapters.
Niles Eldredge, involved in the study of Bolivian trilobites, has found
specimens of the calmoniid trilobite Bouleia which was said to be closely
related to Phacops. After a careful examination of well-preserved individuals,
Eldredge (1980) concluded that the resemblance was entirely superficial
and that Bouleia is an unrelated "homeomorph" of the latter genus, "adapted
to similar environmental demands". He also observed that calmoniid Cryphaeoides
is very similar to dalmanitids. What does this "homeomorphy" mean?
It means "being of the same shape". Typically, morphologically similar
organisms, or homeomorphs, of different systematic position are supposed
to evolve either during the so-called parallel or convergent evolution.
Any morphological similarity between closely related species is commonly
termed parallelism and any morphological similarity between unrelated species
is termed convergence. According to neo-darwinists, in parallelism the
morphological similarity (or the so-called "phenotypic" similarity) results
"from joint possession of independently acquired phenotypic characteristics
produced by a shared genotype inherited from a common ancestor", while
in convergence the similarity results "from joint possession of independently
acquired phenotypic characteristics that are not produced by a genotype
inherited from a common ancestor" (Mayr 1969, p. 202). Of course, the demarcation
between parallelism and convergence is necessarily arbitrary (Webb 1994).
Therefore, the convergence (Batesian and Müllerian mimicry represent
also a case of "convergence") is looked upon as a morphological similarity
which is not based upon the shared genotype and darwinists generally suppose
that it results from similar "selective pressure" in similar environments.
But "similar selective pressure" seems to be a somewhat metaphysical explanation.
Natural selection must operate on random variations but a repeated development
of the same bauplans in different lineages may indicate that certain morphological
solutions are established as "archetypes" (in the sense of Richard Owen
rather than that of Charles Darwin).
The origin of similar species during the parallel and convergent evolution
was discussed also by some structuralists: "According to neo-Darwinists
the phenomenon (parallelism) is due to a combination of two effects, homology
and analogy. Some of the resemblance between, say, a timber wolf and a
Tasmanian wolf, is attributable to the fact that they are descended from
a common ancestor. The cause of the remaining similarity, and especially
of those traits which are found in the two wolves but not in other mammals
of either kind, is that they occupy similar ecological niches. This explanation,
however, depends on the assumption that the two species have been subjected
to very much the same selection pressures in the same sequence, and there
is no evidence for this, nor for a struggle for existence between them
and other variants, with the wolf-type winning in both cases..." (Saunders
and Ho 1984, p. 134).
Really, why only a "similar genotype" and "similar selective pressure"?
To duplicate the process of the evolution of wolf independently in a marsupial
mammal simply by random mutation followed by natural selection seems to
be as metaphysical as a miracle. Why there is no other solution of the
bauplan in the Tasmanian wolf? Is the Darwinian explanation of "optimal
adaptation to common problems" (i.e., that both the placental and marsupial
wolf are built for running and catching) sufficient? Are there some unknown
non-Darwinian forces involved, familiar with the final goal?
And what about similar colours? Are "homeocoloration" and "homeomorphism"
related to different causes? According to the "modern synthesis" every
structure results from random variation and non-random natural selection.
Herein, it seems to be very important to point out that A. R. Wallace (as
a Darwin´s supporter but an opponent of Darwin´s theory of sexual selection)
wrote:
"Yet of all characters this (i.e., colour) is the most difficult to
bring under the laws of utility or of physical connection. Mr. Darwin -
as you are well aware - has shown how wide is the influence of sex on the
intensity of coloration; and he has been led to the conclusion that active
or voluntary sexual selection is one of the chief causes, if not the chief
cause, of all the variety and beauty of colour we see among the higher
animals. This is one of the points on which there is much divergence even
among the supporters of Mr. Darwin, and one as to which I myself differ
from him. I have argued, and still believe, that the need of protection
is a far more efficient cause of variation of colour than is generally
suspected; but there are evidently other causes at work, and one of these
seems to be an influence depending strictly on locality, whose nature we
cannot yet understand, but whose effects are everywhere to be seen when
carefully searched for." and gave us several interesting examples (see
Wallace 1878, p. 254-267).
Of course, the Wallace´s interesting "unknown local action" represents
a designation of "unknown causes of known phenomena" and as such it was
forgotten by evolutionists of the era of "modern synthesis" because the
"appeal to the unknown" was supposed to be metaphysical, not scientific
(see e.g. Simpson 1953, p. 133-134). However, the inexplicable "Local Causes
of Colour-development" were attributed by Wallace (1878, p. 216-217) to
"probably... chemical peculiarities in the soil or vegetation".
Unfortunately, at present, with few exceptions, the view of natural
phenomena is shaped and controlled by opinion-makers and scientific leaders
who are heterodox neo-darwinists. Their worldview still yields a special
picture of the world in which some phenomena, as e.g. the "influence depending
strictly on locality", simply do not exist. But in true science, fact must
yield perception, not the other way around.
Many works are devoted to theoretical explanations of the principles
of camouflage and mimicry but the question remains, however, whether the
most common Darwinian solution is correct.
Bates´s observations were especially invited by Charles Darwin (several
years after his famous first issue of the "Origin of Species" in 1859)
and since Bates´s preliminary study "Contributions to an insect fauna of
the Amazon Valley. Lepidoptera: Heliconidae" (1862) the interpretation
of mimicry has been a sparring ground for supporters and opponents of Darwin´s
Theory of Natural Selection (see e.g. Panchen 1992, p. 270).
The opponents, for example, argued that selection cannot account for
the evolution of mimicry because the latter would be of no "selective advantage"
until well-developed and that the proposed "small steps of increasing selective
advantage" did not really involve in the evolution of mimicry (e.g., Goldschmidt
1945). They also pointed to the fact that what to a human eye may seem
to be a real similarity, e.g. between two different organisms, need not
be such to the eyes of a predator. Thomas Hunt Morgan stressed that the
"so-called mimicry" is "purely imaginary, in the sense that the resemblance
has not been acquired on account of its relation to the animal imitated.
There is no need to question that in some cases animals may be protected
by their resemblance to other animals, but it does not follow, despite
the vigorous assertions of some modern Darwinians, that this imitation
has been the result of selection." (Morgan 1903, p. 358-9).
Almost completely identical views have been proposed in numerous works
of the Austrian zoologist Franz Heikertinger (for illustration see herein
a selected bibliography of his most important articles, Heikertinger 1914-37).
Certainly, the dangers of assuming that a given similarity is mimetic are
great but, on the other hand, there can no longer be a doubt that many
animals are really well-camouflaged in their habitats. Curiously, we are
to face a situation that a century old study by some remarkable darwinists
has shown only, that the very reality of the phenomena of mimicry and camouflage
is still an open question. Recent structuralists (e.g., Edlinger, Gutmann
and Weingarten 1991, and Gutmann, personal communication) give us their
traditional answer that the very phenomena of camouflage and mimicry are
almost poorly imaginary and that for the predator they probably do not
exist. They argue that because predators hunt primarily by smell and hearing,
the coloration of the prey would provide no protection. Some structuralists
also believe that the so-called parallel and convergent evolution are both
inherently inadaptive.
We believe, however, that such a structuralists´ view is oversimplified.
The diet of predators which hunt by sight has been investigated in many
experiments and the significance of camouflage, mimicry and warning coloration
was determined in several serious studies. Moreover, Heráò (1976) has correctly
stressed that even though smell and hearing are important in the orientation
of many predators, visual clues are absolutely necessary for the execution
of the final attack.
Darwinian biologists have discovered that there is really no doubt
that camouflage and mimicry act effectively together with the animals´
other means of defence.
Despite a noteworthy lack of supporting data, on the other hand, for
darwinists, at least for those which believe in the so-called "modern synthesis",
there is their traditional "Theory of Natural Selection" explaining all
parallelisms and convergences, as well as deceptive and protective adaptations
in both predators and prey.
Darwinian biologists have "discovered" that parallelism, convergence,
camouflage and mimicry are all the strongest sort of evidence for the efficacy
of natural selection and its adaptive orientation in evolution. More exactly:
"The Darwinian theory of evolution states that some genotypes in a population
of a given organism have a higher reproductive efficiency than others.
As a result of this greater reproduction, the favored type will increase
in frequency; possibly it will replace the less favored type." (Lewontin
1965).
They believe that natural selection acts as a "Great Unifying Principle"
(including Owen 1980) and see camouflage and mimicry as a kind of a "grand
co-evolutionary arms race" in which predators evolve more efficient ways
to catch prey and the prey evolves better and better ways to escape (of
course, in this philosophy only the best camouflaged prey survives). How
strong are such orthodox views?
We argue that it is still an open question whether there is a "demon"
of Natural Selection responsible for the development of camouflage and
mimicry. Thanks to the American palaeontologist Stephen Jay Gould the neo-Darwinian
"modern synthesis" has broken down on both of its fundamental claims: that
macroevolution is only an extrapolation of microevolution (i.e. of the
gradual allelic substitutions) and that the only force in evolution is
natural selection leading to adaptation (see Gould 1980). There can be
no doubt of Gould´s right to be called "first man" of the present-day evolutionary
theory, at least in the United States. He has written many books and typically
all essays in his books were printed first in the journal Natural History
(published by the American Museum of Natural History). What about his view
of mimicry?
In an interesting essay entitled "A Darwinian Paradox" (Gould 1979,
reprinted in his famous book "The Panda´s Thumb: More Reflections in Natural
History", New York, W. W. Norton 1980) he has discussed an example of mimicry
in the Philippine anglerfish Antennarius maculatus (Antennariidae, Lophiiformes)
described by American ichthyologists Theodore Pietsch and David Grobecker
(see also Grobecker 1981, Pietsch and Grobecker 1978, Pietsch and Grobecker
1990). Anglerfishes are certainly masters of aggressive mimicry among vertebrates
and the mentioned species is characterized by an impressive luring apparatus,
a fishlike bait (esca) placed on the end of a modified anterior spine (illicium)
of its dorsal fin. The anglerfish, approximately 10 cm in length, rests
inert on the bottom, looking like coral-, sponge- and algae-encrusted rock,
moving the illicium (about 2.7 cm long) with the fishlike esca (about 1.4
cm long). The white and chocolate-brown-coloured esca is strongly compressed
laterally bearing extensions simulating caudal, pectoral, pelvic, dorsal
and anal fins. Moreover, this false "fish" is provided with four or five
strongly pigmented vertical bands just behind false eyes (in area corresponding
to the false shoulder and pectoral region) and two lighter bands (in area
of the false caudal fin). So, the bait forms "nearly an exact replica of
a small fish that could easily belong to any of a number of percoid families
common to the Philippine region" (Pietsch and Grobecker 1978, p. 370).
It is important to stress that the movements of the bait improve the mimicry
and that the small reef fishes spotting the bait are captured by the voracious
anglerfish in milliseconds (anglerfishes are fastest predators among vertebrates).
Gould´s solution to the problem of evolution of such an extremely impressive
mimicry is interesting. Gould points to the well-known formula "random
variation followed by natural selection" as well as to the D´Arcy Thompson´s
vision of organisms directly shaped "by physical forces acting upon them".
Stephen Jay Gould wrote:
"Organisms jump suddenly from one optimum to another when the regime
of physical forces alters. We now know that physical forces are too weak,
in most cases, to build form directly - and we look to natural selection
instead. But we are derailed if selection can only act in a patient and
piecemeal way - step by sequential step to build any complex adaptation.
I believe that a solution lies in the essence of Thompson´s insight,
shorn of the unsubstantiated claim that physical forces shape organisms
directly. Complex forms are often built by a much simpler (often a very
simple) system of generating factors. Parts are connected in intricate
ways through growth, and alteration of one part may resound through the
entire organism and change it in a variety of unsuspected ways." (Gould
1979, p. 42)
The above mentioned Gould´s "solution" of the case of aggressive mimicry,
however, tells nothing. It seems to be that it is rather a solution of
the problem of Darwinian theory itself - a "macroevolutionary" improvement
of the synthetic theory. Orthodox neo-darwinists say that macroevolutionary
theory is a non-Darwinian destruction of "modern synthesis", the heterodox
ones that it is "comeback" of true Darwin´s "darwinism" (with Darwin´s
pluralism and his "mysterious laws of growth"), while structuralists are
commonly pleased with Gould´s critique of the adaptationist programme.
But where is any explanation of our case? It is clearly kept away from
us because structuralism and constructional morphology imposed on neo-darwinism
does not seem to be the best way to explain the phenomena of camouflage
and mimicry. It does not seem to be a way at all.
Curiously, it seems that only the "modern synthesis" (and "scientific"
creationism, of course) has "explanatory powers" to give at least a sort
of "solution" in the cases of camouflage and mimicry. Unfortunately, the
explanatory power is based on metaphysical assumptions (see also below).
All the history of the so-called "modern synthesis" shows that we can construct
a theory or rather dogmatic ideology that´s proof against almost any real
evidence. Thus, in darwinism, the idea of simple mechanical selection (of
course, important but destructive and secondary) takes the place of serious
searching for the very origin of camouflage and mimicry. This is both understandable
and unsatisfying: understandable because of the selectionists´ dogmatic
believe in randomness of mutations, and unsatisfying because few (if any!)
of the selectionists´ adaptive scenarios are correct (there is no criterion
of "fitness" other than survival, see esp. Macbeth 1978) over the full
spectrum of the evolutionary problems, particularly in the fossil record.
The orthodox neo-darwinists still argue, for example, that a "key adaptation
arose within a particular group of organisms which enabled that group to
compete and replace a previously dominant group". Such a new group is regarded
as competitively superior or advanced ("higher") to the one (primitive
or "lower") that it replaced. But no evidence for such an assumption is
observable in the fossil record (see especially the study of Benton 1987).
It becomes more and more difficult to understand how the new "random" mutation
arises and how it is that the evolution is so creative.
The great Irish dramatist and winner of the Nobel Prize for Literature
(1925), George Bernard Shaw wrote about the theory of natural selection:
"...what did Darwin really discover? Here, I am afraid, I shall require
once more the assistance of the giraffe, or, as he was called in the days
of the celebrated Buffon, the camelopard (by children, cammyleopard). I
do not remember how this animal imposed himself illustratively on the Evolution
controversy; but there was no getting away from him then; and I am old-fashioned
enough to be unable to get away from him now. How did he come by his long
neck? Lamarck would have said, by wanting to get at the tender leaves high
up on the tree, and trying until he succeeded in wishing the necessary
length of neck into existence. Another answer was also possible: namely,
that some prehistoric stock-breeder, wishing to produce a natural curiosity,
selected the longest-necked animals he could find, and bred from them until
at last an animal with an abnormally long neck was evolved by intentional
selection, just as the race-horse or the fantail pigeon has been evolved.
Both these explanations, you will observe, involve consciousness, will,
design, purpose, either on the part of the animal itself or on the part
of a superior intelligence controlling its destiny. Darwin pointed out
- and this and no more was Darwin´s famous discovery - that a third explanation,
involving neither will nor purpose nor design either in the animal or anyone
else, was on the cards. If your neck is too short to reach your food, you
die. That may be the simple explanation of the fact that all the surviving
animals that feed on foliage have necks or trunks long enough to reach
it. So bang goes your belief that the necks must have been designed to
reach the food. But Lamarck did not believe that the necks were so designed
in the beginning: he believed that the long necks were evolved by wanting
and trying. Not necessarily, said Darwin. Consider the effect on the giraffes
of the natural multiplication of their numbers, as insisted on by Malthus.
Suppose the average height of the foliage-eating animals is four feet,
and that they increase in numbers until a time comes when all the trees
are eaten away to within four feet of the ground. Then the animals who
happen to be an inch or two short of the average will die of starvation.
All the animals who happen to be an inch or so above the average will be
better fed and stronger than the others. They will secure the strongest
and tallest mates; and their progeny will survive whilst the average ones
and the sub-average ones will die out. This process, by which the species
gains, say, an inch in reach, will repeat itself until the giraffe´s neck
is so long that he can always find food enough within his reach, at which
point, of course, the selective process stops and the length of the giraffe´s
neck stops with it. Otherwise, he would grow until he could browse off
the trees in the moon. And this, mark you, without the intervention of
any stock-breeder, human or devine, and without will, purpose, design,
or even consciousness beyond the blind will to satisfy hunger. It is true
that this blind will, being in effect a will to live, gives away the whole
case; but still, as compared to the open-eyed intelligent wanting and trying
of Lamarck, the Darwinian process may be described as a chapter of accidents.
As such, it seems simple, because you do not at first realize all that
involves. But when its whole significance dawns on you, your heart sinks
into a heap of sand within you. There is a hideous fatalism about it, a
ghastly and damnable reduction of beauty and intelligence, of strength
and purpose, of honor and aspiration, to such casually picturesque changes
as an avalanche may make in landscape, or a railway accident in a human
figure. To call this Natural Selection is a blasphemy, possible to many
for whom Nature is nothing but a casual aggregation of inert and dead matter,
but eternally impossible to the spirits and souls of the righteous. If
it be no blasphemy, but a truth of science, then the stars of heaven, the
showers and dew, the winter and summer, the fire and heat, the mountains
and hills, may no longer be called to exalt the Lord with us by praise:
their work is to modify all things by blindly starving and murdering everything
that is not lucky enough to survive in the universal struggle for hogwash."
(Shaw 1921, p. xliv-xlvi).
We maintain that the Darwinian explanations take too narrow a view
of the phenomena of camouflage and mimicry as a whole and that they tend
to become one-sided, strongly overemphasizing the role of selection. There
is an almost infinite array of interesting features of the fossil record
(and of the living things, too) that could (and should) be more systematically
exploited. The Darwinian reductionists´ agenda cannot be complete until
we know at least something about the origin of "bauplan similarities" (not
only about their preservation which is self-evident from the preservation).
An understanding of the origin of bauplans is inseparable from the most
important evolutionary questions. It is impossible to conduct a meaningful
search for the so-called "mechanisms of evolution" without knowing more
about the circumstances under which the phenomena of mimicry, camouflage,
parallelism, convergence or Wallace´s "locality influence" originated.
It is not surprising that the latter is generally ignored or is supposed
to be poorly accidental because it does not fit the neo-Darwinian schemes.
Therefore, we point to the obvious paradox. Darwinians systematically
overlook the very mechanisms that may have led to the emergence of any
bauplan similarity. How, on the other hand, do they decide that a bauplan
has a "fitness"? Of course, by the fact that it survives. But again, it
is self-evident that a bauplan that survives or is repeated in the course
of the history must be useful.
The well-known English sci-fi writer, Arthur C. Clarke, under the influence
of the theory of natural selection, wrote (first words of his greatest
space adventure "2001: A Space Odyssey") about the evolution of mankind:
"The drought had lasted now for ten million years, and the reign of the
terrible lizards had long since ended. Here on the Equator, in the continent
which would one day be known as Africa, the battle for existence had reached
a new climax of ferocity, and the victor was not yet in sight. In this
barren and desiccated land only the small or the swift or the fierce could
flourish, or even hope to survive..." (Clarke 1968) but, on the other hand,
the very responsibility for the origin of man he clearly ascribed to "T.M.A.-1"
constructed by intelligent extraterrestrials. Why?
It is probably because the author was unable to imagine the origin
of man´s intelligence as a result of infinite random variations stabilized
by natural selection (orthodox neo-Darwinian view). On the contrary, members
of the relatively newly established school of macroevolutionists (from
this point of view we may say "Darwinian structuralists") are at least
convinced that many developmental constraints impose important limitations
on the evolution by natural selection (see especially the classical "Critique
of the adaptationist programme" by Gould and Lewontin 1979, reprinted in
1984) and that it does not seem to be reasonable to assume that variations
are infinite. This provided an important insight into the old "modern synthesis".
Of course, also in the ideas of the very heterogeneous movement of contructional
morphologists (see especially their large symposium volume edited by Schmidt-Kittler
and Vogel 1991) there is clearly stressed that in nature the structural
possibilities are limited. White, Michaux and Lambert (1990) have pointed
to Darwin´s acknowledgement that there are some impossible forms.
The latter authors have also remembered two arguments of Hans Driesch
(Driesch 1929) that:"... natural selection, to some degree, is self-evident;
at least as far as it simply states that what is incompatible with permanent
existence cannot exist permanently", and "In speaking of an "explanation"
of the origin of the living specific forms by natural selection, one ...
confuses the sufficient reason for the non-existence of what there is not,
with the sufficient reason for the existence of what there is." White,
Michaux and Lambert (1990) continue that "... natural selection is not
even a sufficient reason for "the non-existence of what is not, " because
some forms may not exist because they are not possible" (the latter authors
have also argued that the range of impossible forms is potentially infinite!).
A modern structuralist´s view of the origin of a bauplan may be expressed
in words of the famous British biologist and an expert in the so-called
morphogenetic fields, Brian Goodwin: "... there is evidence from the study
of complex nonlinear systems describing morphogenetic processes that these
have an intrinsic robustness that gives resultant structures the status
of generic forms, arising within large attractors in morphospace..." (Goodwin
1994). It must be stressed that for all modern structuralists, "genes are
not sufficient causes of biological form any more than last year´s elliptical
passage of the earth around the sun is a sufficient cause of this year´s
elliptical trajectory." (Goodwin 1984).
Structuralists Nijhout (1978) and Saunders and Ho (1984) have argued
that for one butterfly to mimic another requires nothing more than a few
minor alterations and that the patterns on butterflies´ wings are far less
complex than they look. The latter authors have expected similar arguments
to apply in other cases of mimicry as well. They also pointed out that
the advantage due to the Batesian mimicry is density dependent. It has
a value only when the mimic is comparatively rare. The more Batesian mimics
there are the more predators will learn to associate the mimics with a
palatable prey.
Yes, the latter structuralists´ arguments are important but, on the
other hand, they represent a reduction again, overlooking the ecosystem.
Analogically, why the structuralists do not ask, for example "Why there
is a predator-prey system which oscillates around an equilibrium?". Possibly
because they agree with neo-darwinists that the latter is set by external
limits to the prey population? But there is an intrinsic rule of predator-prey
stability in which predators increase or decrease their own numbers and
rates of food consumption in proportion to prey density. We may ask as
well: "Which was the first: the prey overpopulation or the ability of foxes
to produce more offspring in the years of prey increase?"
It is obvious that we must replace the structuralists´ reductionism
by a form of non-Darwinian holism.
It must be stressed that a majority of naturalists do not know exactly
what it is the word "holism" (as well as its counterpart "reductionism").
Possibly the best analysis of the usage of the term was given by WILSON
(1988) who has identified three different senses of holism:
1. Mechanistic holism, i.e. a "subdivision" of holism with simple and
very common tendency to explain a phenomenon with many (not only one) "factors".
From this point of view (i.e. view of complexity) a great majority of naturalists
are holists.
2. Descriptive holism, i.e. holism as a practical tool for making predictions.
This kind says nothing about causal relationships among the parts. It is
interesting that in many and many cases such a holism seems to be the only
route to true understanding. Herein, there is embedded also the essence
of the third type of holism: "...population problems may best be approached
by starting with the ecosystem and working down rather than by starting
with the individual or species and working up as is usually done." (E.
P. Odum 1959). From this point of view (i.e. view of practicality) a majority
of naturalists are reductionists because they point to the fact that practicality
is not an explanation itself.
3. "Metaphysical" holism, i.e. true holism which denies that higher-level
processes of a hierarchy are simply caused by (or fully explicable in terms
of) lower-level processes. The most important issue of this is that higher
levels of a hierarchy (or integrated wholes) impose order on lower levels
(i.e. on their parts). This is also called "downward causation" and is
commonly used also in a Darwinian sense (i.e. in relation to natural selection
which is "imposed" on the organism from the "outside"), see e.g. Campbell
(1974).
Keeping in mind Karl Popper´s "Darwinism as a metaphysical research
program" (1974, and "Unended Quest" 1974, 1976), we have a second reason
to take selectionism as "metaphysical" (the first one is that the theory
of natural selection is capable, on poorly theoretical basis, to "explain"
every thinkable structure, every thinkable morphology or physiology of
every individual organism, every state of ecosystem, etc.).
Of course, the best way for the present authors is to use the second
"subdivision" of holism because it is practical. However, at least in this
general part, it is inevitable to touch the really "metaphysical" issues.
All darwinists must agree that the camouflage-related and mimetic structures
of a given animal represent a self-evident case of "downward causations"
(downward from the ecosystems to the animals). Therefore, all darwinists
are holists (looking not only for the "upward causation") while many structuralists
are reductionists (many of them stress only the so-called "internal factors"
of the animal´s structure without connection with the ecosystem). We argue,
however, that the Darwinian holism is incorrect especially from the metaphysical
point of view, i.e. incorrect in its vision of "nature red in tooth and
claw" - overwhelming competition and strong selective pressures as the
only "non-random factors of evolution". Such a kind of world is impossible
because of its inability to produce organic diversity (Rosen 1978) and
even survive. It would be useful to define how we are taking holism herein
because non-holistic functionalists´ and structuralists´ speculations bring
us to an old question: "Which was the first: the chicken or the egg?" and
we are back to where we started.
The question is whether there is anything overlooked in the ecosystem
theories. It seems that there is really amazingly little known about the
very function of a whole ecosystem because the commonly used argument against
any concept of superorganism is that there are not only "symbioses" in
ecosystems but also many cases of predation. But this is clearly a misunderstanding
because the latter constitutes one of the most important forms of ecosystem
regulation. We may remember herein at least the so-called Energy Quality
Theory of the famous ecologist Howard T. Odum saying that: "all units in
systems are symbiotic (mutually contributing to the others´ survival, many
species (and other kinds of units) have evolved very close mutual relationships
wherein pairs operate almost as a single unit, each requiring the other."
(see H. T. Odum 1983, p. 394-395).
Very important view of inseparable interrelatedness of living organisms
and their abiotic environment we find in the classical and possibly the
most enjoyed textbook on ecology by Eugene P. Odum: "The ecosystem is the
basic functional unit in ecology, since it includes both organisms (biotic
communities) and abiotic environment, each influencing the properties of
the other and both necessary for maintenance of life as we have it on the
earth." (E. P. Odum 1971, p. 8).
Lewis Thomas, a well-known American writer, winner of the National
Book Award in 1974 for his collection of essays "The Lives of a Cell",
wrote about the life in the sea:
"Perhaps something is wrong in the way we look at them (i.e., living
things). From our distance we see them as separate, independent creatures
interminably wrangling, as a writhing arrangement of solitary adversaries
bent on killing each other. Success in such a system would have to mean
more than mere survival; to make sense, the fittest would surely have to
end up standing triumphantly alone. This, in the conventional view, would
be
the way of the world, the ultimate observance of nature s law. It was to
delineate such a state of affairs that the hideous nineteenth-century phrase,
´Nature red in tooth and claw,´ was hammered out.
What is wrong with this view is that it never seems to turn out that
way. There is in the sea a symmetry, a balance, and something like the
sense of permanence encountered in a well-tended garden." (Thomas 1971).
In the ecosystems there is a kind of an intrinsic homeostasis (i.e.
self-maintenance and self-regulation) present. The order is a self-evident
one and the existence of the living world throughout its long history is
both a tremendous puzzle to us as well as a self-justification of it. As
pointed out by Austin Hobbart Clark: "The more we learn of the world in
which we live the more clearly do we see that an orderly and definite plan
underlies and dominates all the phenomena of nature. The living world of
animals and plants is no exception. It is not chaotic. To picture it as
chaotic is simply to confess our ignorance..." (Clark 1930, p. 208).
We are impressed by W. I. Vernadsky (e.g. 1929, 1945) followed by E.
P. Odum (1971) who raised the interesting possibility that individual organisms
"not only adapt to the physical environment, but by their concerted action
in ecosystems they also adapt the geochemical environment to their biological
needs." The latter view is also retained in the well-known (and at present
days very discussed) Gaia theory (i.e., theory of the Earth as a superorganism)
of James Lovelock (see especially his books, Lovelock 1979, 1988, 1994)
as well as in the viewpoints of English-born Zen-philosopher Alan Watts
who enjoyed popularity in San Francisco at least during the 1950s. In both
the latter cases the Earth is taken as a whole. Both show that, although
it is common to speak of the Earth as of a planet inhabited by life, it
may be confusing. Beginning rather crudely, we may view the living things
having generally very short lives and being completely dependent on the
surrounding "abiological" environment. In fact, this is not the way it
is but the way we see it due to our reduction because life itself is as
old as the Earth´s record of its crust, water, and sedimentary rocks (see
any recent textbook on historical geology). Therefore, we may say that
the Earth "implies" Life as well as the Life "implies" Earth. Really, there
is not only the question "How does the planet control its life?" but also
"How does the life control its planet?".
"Ecologists often speak of the "evolution of environments" over and
above the evolution of organisms. For man did not appear on earth until
the earth itself, together with all its biological forms, had evolved to
a certain degree of balance and complexity. At this point of evolution
the earth "implied" man, just as the existence of man implies that sort
of a planet at that stage of evolution... But, as Douglas E. Harding has
pointed out, we tend to think of this planet as a life-infested rock, which
is as absurd as thinking of the human body as a cell-infested skeleton.
Surely all forms of life, including man, must be understood as "symptoms"
of the earth, the solar system, and the galaxy - in which case we cannot
escape the conclusion that the galaxy is intelligent..." (Watts 1966, reprint
1970).
Therefore, we believe that whatever is underlying all these regulating
phenomena, whether mimicry, warning coloration, camouflage, mutualism,
parasitism or predator-prey relationships, it is going to turn out to be
universal. But the latter does not mean that it is anything analogical
to the poorly metaphysical "basic evolutionary mechanism" but rather to
an intrinsic and really holistic developmental control of the gigantic
planetary superorganism itself. Living world is much richer than all sorts
of neo-Darwinian theories are portraying it to be and development seems
to be a better word than "evolution".
Written by James Lovelock: "I admit that we have often been provocative.
We had to be or our work would have been ignored. I now realize that our
provocation was a mistake. Nothing we could say or offer as evidence would
convince the closed minds of our opponents. In the case of discoveries
such as the global distribution of the chlorofluorocarbons and the finding
that dimethyl sulfide is the natural sulfur carrier, I have found that
the initial scorn and rejection was soon followed by the development of
the topic as a major scientific interest. To the real scientists I say,
be patient and don´t knock Gaia too hard; before long she may be paying
your grants." (Lovelock 1991).
We thank Dr. Rudolf Prokop (National Museum), Dr. Oldøich
Fatka and Doc. Jaroslav Marek (Charles University) for loaning of some
interesting literature. We are indebted to Dr. Rudolf Prokop, Dr. Oldøich
Fatka, Dr. Vojtìch Turek and Dr. Radvan Horný (National Museum) for their
very important comments (although sometimes sceptical). We thank Dr. Rudolf
Prokop for his invaluable help with and discussions about the type material
deposited in collections of the Department of Palaeontology, National Museum.
Finally, we wish to express our deepest gratitude to Prof. Ivo Chlupáè
(Charles University) for his generous gift of the photographs presented
herein, for literature, invaluable informations and especially for his
most encouraging comments.
ARISTOTLE (4th century B. C.): Historia Animalium, Engl. transl. History of Animals, Transl. by R. Crosswell, 1883, 326 pp. London.
BABCOCK, L. E. (1982): Original and diagenetic color patterns in two phacopid trilobites from the Devonian of New York. - Proceedings of the Third North American Paleontological Convention, 1: 17-22.
BABCOCK, L. E. (1993a): Trilobite malformations and the fossil record of behavioral asymmetry. - Journal of Paleontology, 67(2): 217-229.
BABCOCK, L. E. (1993b): The Right and the Sinister. - Natural History, 102(7): 32-39. New York.
BABCOCK, L. E. - ROBISON, R. A. (1989): Preferences of Palaeozoic predators. - Nature, 337(6209): 695-696. London.
BATES, H. W. (1862): Contributions to an insect fauna of the Amazon Valley. Lepidoptera: Heliconidae. - Transactions of the Linnean Society, 23: 495-566. London.
BATES, H. W. (1892): The Naturalist on the River Amazons (1st Ed. The Naturalist on the River Amazons, a Record of Adventure, Habits of Animals, Sketches of Brazilian and Indian Life... during Eleven Years of Travel, 1863) , with an appreciation by Charles Darwin (p. vii-xiii), 407 pp., J. M. Dent & Sons Ltd., London and E. P. Dutton & Co., New York.
BECKER, H. F. (1965): Flowers, Insects, and Evolution: Specialization developed from mutual adaptations. - Natural History, 74(2): 38-45. New York.
BENTON, M. J. (1987): Progress and Competition in Macroevolution. - Biological Reviews, 62: 305-338. Cambridge (U.K.).
BERGSTRÖM, J. (1973): Organization, life, and systematics of trilobites. - Fossils and Strata, 2: 1-69. Oslo.
BOUCOT, A. J. (1981): Principles of Benthic Marine Paleoecology. 463 pp., Academic Press. New York.
BOUCOT, A. J. (1990): Evolutionary Paleobiology of Behavior and Coevolution. 725 pp., Elsevier. Amsterdam, Oxford, New York, Tokyo.
BROWER, L. P. - BROWER, J. V. Z. (1962): Investigations Into Mimicry. - Natural History, 71(4): 8-19. New York.
CAMPBELL, D. T. (1974): Unjustified Variation and Selective Retention in Scientific Discovery, p. 139-161. In: F. J. AYALA & Th. DOBZHANSKY (Eds.): Studies in the Philosophy of Biology: Reduction and Related Problems, 390 pp., The Macmillan Press Ltd. London, Basingstoke, New York, Dublin, Melbourne, Johannesburg, Madras.
CARPENTER, G. D. H. (1921): Experiments of the relative edibility of insects with special reference to coloration. - Transactions of the Entomological Society London, 1921: 1-105. London.
CARPENTER, G. D. H. (1941): The relative frequency of beak-marks on butterflies of different edibility to birds. - Proceedings of the Zoological Society of London, Series A, 111: 223-231. London.
CHATTERTON, B. D. E. - SIVETER, D. J. - EDGECOMBE, G. D. - HUNT, A. S. (1990): Larvae and relationships of the Calymenina (Trilobita). - Journal of Paleontology, 64(2): 255-277. Lawrence.
CHLUPÁÈ, I. (1970): Phyllocarid crustaceans of the Bohemian Ordovician. - Sborník geologických vìd, Paleontologie, 12: 41-77. Praha.
CHLUPÁÈ, I. (1977): The phacopid trilobites of the Silurian and Devonian of Czechoslovakia. - Rozpravy Ústøedního ústavu geologického, 43: 1-172. Praha.
CLARK, A. H. (1930): The New Evolution: Zoogenesis. 297 pp., Bailliere, Tindall & Cox. London.
CLARKE, A. C. (1968): 2001: A Space Odyssey, based on the screenplay by Arthur C. CLARKE and Stanley KUBRICK (First published in Great Britain by Hutchinson, 1968), New Edition 1990, 266 pp., Legend, Arrow Books Ltd.
COTT, H. B. (1940): Adaptive Colouration in Animals, 508 pp., Methuen. London.
COWEN, R. - GERTMAN, R. - WIGGINS, G. (1973): Camouflage patterns in Nautilus, and their implications for cephalopod paleobiology. - Lethaia, 6(2): 201-214. Oslo.
CURIO, E. (1965): Ein Falter mit "falschem Kopf" (Ergebnisee der Deutschen Galápagos-Expedition 1962/63. XII). - Natur und Museum, 95(2): 43-46. Frankfurt a. M.
DARWIN, Ch. (1859): On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life, 703 pp., John Murray, London.
DIAMOND, J. M. (1994): Two-faced mimicry. - Nature, 367(6465): 683-684. London.
DOBZHANSKY, Th. (1951): Genetics and the Origin of Species. 3rd Edition, 364 pp., Columbia Univ. Press. New York, London.
DRIESCH, H. (1929): The science and philosophy of the organism, 344 pp., A. & C. Black Ltd. London.
EDGECOMBE, G. D. - SPEYER, S. E. - CHATTERTON, B. D. E. (1988): Protaspid larvae and phylogenetics of encrinurid trilobites. - Journal of Paleontology, 62(5): 779-799. Lawrence.
ELDREDGE, N. (1971): The allopatric model and phylogeny in Paleozoic invertebrates. - Evolution, 25(1): 156-167. Lawrence.
ELDREDGE, N. (1974): Stability, diversity, and speciation in Paleozoic epeiric seas. - Journal of Paleontology, 48(3): 540-548. Lawrence.
ELDREDGE, N. (1980): An Extravagance of Species: The diversity of fossil trilobites poses a challenge to traditional evolutionary theory. - Natural History, 89(7): 46-51. New York.
ELDREDGE, N. (1984): Simpson´s Inverse: Bradytely and the Phenomenon of Living Fossils, p. 272-277. In: N. ELDREDGE & S. M. STANLEY (Eds.): Living Fossils. 291 pp., Springer Verlag, New York, Berlin, Heidelberg, Tokyo.
ELDREDGE, N. (1990): Hierarchy and Macroevolution, p. 124-129. In: D. E. G. BRIGGS & P. R. CROWTHER (Eds.): Palaeobiology: A Synthesis. 583 pp., Blackwell Scientific Publ. Oxford.
ELDREDGE, N. - GOULD, S. J. (1972): Punctuated Equilibria: An Alternative to Phyletic Gradualism. p. 82-115. In: T. J. M. SCHOPF (Ed.): Models in Paleobiology, 250 pp.. Freeman, Cooper & Co., San Francisco.
ESKER, G. C., III (1968): Color markings in Phacops and Greenops from the Devonian of New York. - Palaeontology, 11: 498. London.
FOOTE, M. (1990): Nearest-neighbor analysis of trilobite morphospace. - Systematic Zoology, 39(4): 371-382. Lawrence.
FRICKE, H. W. (1975): The role of behaviour in marine symbiotic animals, p. 581-594. In: Symposia of the Society for Experimental Biology, 29: Symbiosis, 633 pp., Cambridge University Press for the Society for Experimental Biology. Cambridge (England), London, New York, Melbourne.
GARRETSON, M. W. (1953): Color in trilobites of Trenton age. - Science, 117: 17. Washington, D. C.
GHISELIN, M. T. (1969): The Triumph of the Darwinian Method, 287 pp., University of California Press. Berkeley, Los Angeles.
GOLDSCHMIDT, R. B. (1945): Mimetic polymorphism: A controversial chapter of Darwinism.- Quaterly Review of Biology, 20: 147-164, 205-230.
GOODWIN, B. C. (1984): Changing from an evolutionary to a generative paradigm in biology, p. 99-120. In: J. W. POLLARD (Ed.): Evolutionary Theory: Paths into the Future. 271 pp., Willey. Chichester, New York, Brisbane, Toronto, Singapore.
GOODWIN, B. C. (1994): Homology, Development, and Heredity, p. 229-247. In: B. K. HALL (Ed.): Homology: The Hierarchical Basis of Comparative Biology, 483 pp., Academic Press. San Diego, New York, Boston, London, Sydney, Tokyo, Toronto.
GOTTFRIED, M. D. (1989): Earliest Fossil Evidence for Protective Pigmentation in an Actinopterygian Fish. - Historical Biology, 3(1): 79-83. Chur, London, Paris, New York, Melbourne.
GOULD, S. J. (1979): A Darwinian Paradox. - Natural History, 88(1): 32, 36, 40, 42, 44. New York.
GOULD, S. J. (1980): Is a new and general theory of evolution emerging? - Paleobiology, 6(1): 119-130. Ithaca.
GOULD, S. J. (1989): Wonderful Life: Burgess Shale and the Nature of History, 347 pp., W. W. Norton. New York, London.
GOULD, S. J. - ELDREDGE, N. (1977): Punctuated equilibria: the tempo and mode of evolution reconsidered. - Paleobiology, 3(2): 115-151. Ithaca.
GOULD, S. J. - LEWONTIN, R. C. (1979): The Spandrels of San Marco and the Panglossian Paradigm: A Critique of the Adaptationist Programme. - Proceedings of the Royal Society of London, B, 205: 581-598. London.
GOULD, S. J. - LEWONTIN, R. C. (1984): The Spandrels of San Marco and the Panglossian Paradigm: A Critique of the Adaptationist Programme. p. 252-270. In: E. SOBER (Ed.): Conceptual Issues in Evolutionary Biology: An Anthology. 725 pp., Bradford Book. Cambridge (Massachusetts), London.
GREENE, H. W. - McDIARMID, R. W. (1981): Coral Snake Mimicry: Does It Occur? - Science, 213(4513): 1207-1212. Washington, D. C.
GROBECKER, D. B. (1981): Steady as a Rock, Fast as Lightning (photographs by D. B. GROBECKER). - Natural History, 90(5): 50-53. New York.
HARRINGTON, H. J. (1959): General Description of Trilobita, O38-O117. In: R. C. MOORE (Ed.): Treatise on Invertebrate Paleontology, Part O, Arthropoda 1. Geological Society of America, Boulder, and University of Kansas Press, Lawrence (Kansas).
HEIKERTINGER, F. (1914): Über die beschrankte Wirksamkeit der natürlichen Schutzmittel der Pflanzen gegen Tierfraßs. - Biologisches Zentralblatt, 34(2): 81-108. Leipzig.
HEIKERTINGER, F. (1915): Die Frage von den natürlichen Pflanzenschutzmitteln gegen Tierfraßs und ihre Lösung. - Biologisches Zentralblatt, 35(6-7): 257-281. Leipzig.
HEIKERTINGER, F. (1917a): Das Scheinproblem von der Zweckmäßsigkeit im Organischen: Ein Beitrag zur Kritik selektionstheoretischer Probleme. - Biologisches Zentralblatt, 37(7): 333-352. Leipzig.
HEIKERTINGER, F. (1917b): Über einige Versuche mit Lytta vesicatoria L. zur selektionistischen "Schutzmittel"-Frage. - Biologisches Zentralblatt, 37(9): 446-460. Leipzig.
HEIKERTINGER, F. (1919a): Die metöke Myrmekoidie. Tatsachenmaterial zur Lösung des Mimikryproblems. - Biologisches Zentralblatt, 39(2): 65-102. Leipzig.
HEIKERTINGER, F. (1919b): Versuche und Freilandforschungen zur Mimikryhypothese. - Biologisches Zentralblatt, 39(8): 352-363. Leipzig.
HEIKERTINGER, F. (1921): Welchen Quellen entspringen die biologischen Trachthypothesen? I. H. W. Bates. - Zoologischer Anzeiger, 53(11/13): 286-297. Leipzig.
HEIKERTINGER, F. (1922a): Welchen Quellen entspringen die biologischen Trachthypothesen? II. A. R. Wallace. - Zoologischer Anzeiger, 54(1/2): 30-38. Leipzig.
HEIKERTINGER, F. (1922b): Welchen Quellen entspringen die biologischen Trachthypothesen? III. A. R. Wallace (Die Warntrachthypothese). - Zoologischer Anzeiger, 54(1/2): 39-47. Leipzig.
HEIKERTINGER, F. (1922c): Welchen Quellen entspringen die biologischen Trachthypothesen? IV. Roland Trimen. - Zoologischer Anzeiger, 54(7/8): 177-184. Leipzig.
HEIKERTINGER, F. (1922d): Welchen Quellen entspringen die biologischen Trachthypothesen? V. Fritz Müller. - Zoologischer Anzeiger, 54(7/8): 185-190. Leipzig.
HEIKERTINGER, F. (1922e): Welchen Quellen entspringen die biologischen Trachthypothesen? - VI. Die Schrecktrachthypothese. - Zoologischer Anzeiger, 55(1/2): 1-10. Leipzig.
HEIKERTINGER, F. (1922f): Welchen Quellen entspringen die biologischen Trachthypothesen? - VII. Ch. Darwin (Die Sexualselection). - Zoologischer Anzeiger, 55(7/8): 141-154. Leipzig.
HEIKERTINGER, F. (1922g): Sind die Wanzen (Hemiptera heteroptera) durch Ekelgeruch geschatzt?. Beobachtungen und Versuche auf dem Gebiete der Tiertrachthypothesen. - Biologisches Zentralblatt, 42(10/11): 441-464. Leipzig.
HEIKERTINGER, F. (1923): Ist die "Bekömmlichkeit" tatsächlich das Grundprinzip in der Tierernährung? - Zoologischer Anzeiger, 55(11/13): 248-258. Leipzig.
HEIKERTINGER, F. (1925a): Kann Mimikry durch Selection entstehen? Eine prinzipielle Untersuchung. - Zeitschrift für Morphologie and Ökologie der Tiere, 4(4): 598-614. Berlin.
HEIKERTINGER, F. (1925b): Über die Begriffe "Mimikry" und "Mimese" mit besonderer Berücksichtigung der Myrmekoidie: Zugleich eine Antwort an E. Wasmann. - Biologisches Zentralblatt, 45(5): 272-289. Leipzig.
HEIKERTINGER, F. (1925c): Die Ameisenmimese, I. Die Gesichtsmimese. - Biologisches Zentralblatt, 45(12): 705-727. Leipzig.
HEIKERTINGER, F. (1926a): Die Ameisenmimese, II. Die "gesetzmäßige" Färbungsübereinstimmung zwischen Gast und Wirt (Isochromie). - Biologisches Zentralblatt, 46(6): 351-382, 46(7): 385-405. Leipzig.
HEIKERTINGER, F. (1926b): Die Ameisenmimese, III. Die Tastmimese. - Biologisches Zentralblatt, 46(10): 593-625. Leipzig.
HEIKERTINGER, F. (1927): Die Ameisenmimese, IV. Die Loesung des Problems. - Biologisches Zentralblatt, 47(8): 462-501. Leipzig.
HEIKERTINGER, F. (1930): Über "transformative Schutzfärbung" und ihre wissenschaftliche Begründung. - Biologisches Zentralblatt, 50(4): 193-219. Leipzig.
HEIKERTINGER, F. (1932a): Die Coccinelliden, ihr "Elkelblutt", ihre Warntracht und ihre Feinde. Spezielle Untersuchungen zu allgemein-ökologischen Problemen. - Biologisches Zentralblatt, 52(2): 65-102. Leipzig.
HEIKERTINGER, F. (1932b): Die Coccinelliden, ihr "Elkelblutt", ihre Warntracht und ihre Feinde. Spezielle Untersuchungen zu allgemein-ökologischen Problemen. II. Teil: Die Feinde der Coccineliden. - Biologisches Zentralblatt, 52(7): 385-412. Leipzig.
HEIKERTINGER, F. (1933): Das Rätsel des Papilio dardanus und seine Lösung (Kritik der Schmetterlingsmimikry I.). - Biologisches Zentralblatt, 53(11/12): 561-590. Leipzig.
HEIKERTINGER, F. (1934): Die Mimikrytypen der afrikanischen Papilioniden und ihr Verhältnis zu den nichtmimetischen Verwandten (Kritik der Schmetterlingsmimikry II.). - Biologisches Zentralblatt, 54(7/8): 365-389. Leipzig.
HEIKERTINGER, F. (1935): Die Mimikrytypen der afrikanischen Nymphaliden und ihr Verhältnis zu den nichtmimetischen Verwandten (Kritik der Schmetterlingsmimikry III.). - Biologisches Zentralblatt, 55(9/10): 461-483. Leipzig.
HEIKERTINGER, F. (1936a): Die Mimikrymodelle der Tagfalter Afrikas und ihr "Ekelgeschamck" (Kritik der Schmetterlingsmimikry IV.). - Biologisches Zentralblatt, 56(3/4): 151-166. Leipzig.
HEIKERTINGER, F. (1936b): Die Mimikrymodelle der Tagfalter Afrikas und ihr "Ekelgeschamck" (Kritik der Schmetterlingsmimikry V.). Werden die Tagfalter von Vögeln gejagt? - Biologisches Zentralblatt, 56(9/10): 463-494. Leipzig.
HEIKERTINGER, F. (1937a): Die Mimikrymodelle der Tagfalter Afrikas und ihr "Ekelgeschamck" (Kritik der Schmetterlingsmimikry V.). Werden die Tagfalter von Vögeln gejagt? Teil II: Mageninhaltsuntersuchungen. - Biologisches Zentralblatt, 57(1/2): 2-21. Leipzig.
HEIKERTINGER, F. (1937b): Über zwei Grundbegriffe der Ernährungsbiologie: "Normalnahrungskreis" und "wirkliche Feinde". - Biologisches Zentralblatt, 57(7/8): 431-441. Leipzig.
HEIKERTINGER, F. (1937c): Die Mimikry der Tagfalter Südamerikas: Die Dismorphiinen (Kritik der Schmetterlingsmimikry VI.). - Verhandlungen der Zoologisch-Botanischen Gesellschaft in Wien, 86/87 (for 1936/37): 35-72. Wien.
HERÁÒ, I. (1976): Animal Coloration: The nature and purpose of colours in vertebrates, 158 pp., Hamlyn. London, New York, Sydney, Toronto
HESSLER, R. R. (1965): Lower Mississippian trilobites of the Family Proetidae in the United States, Part II. - Journal of Paleontology, 39(2): 248-264. Menasha.
HUGHES, N. C. (1991): Morphological plasticity and genetic flexibility in a Cambrian trilobite. - Geology, 19(9): 913-916. Boulder.
JABLONSKI. D. - BOTTJER, D. J. (1991): Environmental Patterns in the Origins of Higher Taxa: The Post-Paleozoic Fossil Record. - Science, 252(5014): 1831-1833. Washington, D. C.
JOHNSON, A. L. A. (1981): Detection of ecophenotypic variation in fossils and its application to a Jurassic scallop. - Lethaia, 14(4): 277-285. Oslo.
KÁCHA, P. - ARIÈ, R. (1991): Ontogeny of the trilobite Kosovopeltis svobodai NAJDR from the Bohemian Silurian. - Vìstník Ústøedního ústavu geologického, 66(5): 257-273. Praha.
KÁCHA, P. - ARIÈ, R. (1994): "Species" Protoscutellum simulans (BARRANDE, 1852) is identical to the species Planiscutellum planum (HAWLE et CORDA, 1847) (Styginidae, Trilobita). - Bulletin of the Czech Geological Survey, 69(4): 61-63. Praha.
KETTLEWELL, H. B. D. (1959): Darwin´s Missing Evidence. - Scientific American, 200(3): 48-53. New York.
KIRBY, W. - SPENCE, W. (1817): An Introduction to Entomology, I, II, 530 pp. London.
KOBLUK, D. R. - HALL, R. L. (1976): Preserved colour patterns in Ormoceras westonense from the Middle Ordovician of Quebec. - Canadian Journal of Earth Sciences, 13(10): 1479-1481. Ottawa.
KOBLUK, D. R. - MAPES, R. H. (1989): The Fossil Record, Function, and Possible Origins of Shell Color Patterns in Paleozoic Marine Invertebrates. - Palaios, 4(1): 63-85. SEPM, Tulsa, and Allen Press, Lawrence.
KØÍ, J. - LUKE, P. (1974): Color patterns on Silurian Platyceras and Devonian Merista from the Barrandian area, Bohemia, Czechoslovakia. - Journal of Paleontology, 48(1): 41-48. Tulsa.
KUHN, Th. S. (1962): The Structure of Scientific Revolutions, University of Chicago Press. Chicago.
KUHN, Th. S. (1970): The Structure of Scientific Revolutions, 2nd Enlarged Edition, 172 pp., New American Library. New York.
LAMONT, A. (1967): Environmental significance of eye-reduction in trilobites and recent arthropods: additional remarks. - Marine Geology, 5: 377-378. Amsterdam.
LAMONT, A. (1969): Prolegomena to aggressive mimicry and protective resemblance in early fishes, chelicerates, trilobites and brachiopods. - Scottish Journal of Science, 1(2): 75-103.
LANE, F. W. (1957): Kingdom of the Octopus: The Life-History of the Cephalopoda, 287 pp., Jarrolds. London.
LEWONTIN, R. C. (1965): The Role of Competition in Determining the Intensity of Natural Selection, p. 108-115. In: Research Problems in Biology: Investigations for Students, Series Three, 210 pp. Anchor Books, Doubleday & Company, Inc. Garden City, New York.
LOVELOCK, J. E. (1979): Gaia: A New Look at Life on Earth, 157 pp., Oxford University Press. Oxford.
LOVELOCK, J. E. (1988): The Ages of Gaia: A Biography of Our Living Earth, 253 pp., W. W. Norton and Oxford University Press, New York.
LOVELOCK, J. E. (1991): Toujours Gaia. - Science, 252(5012): 1472. Washington, D. C.
LOVELOCK, J. E. (1994): Gaia: ivoucí planeta, (with the author´s foreword to the Czech edition), translated from "The Ages of Gaia: A Biography of Our Living Earth", New York 1988, 221 pp., Mladá Fronta (Edice Kolumbus), Ministerstvo ivotního prostøedí Èeské republiky. Praha.
MACBETH, N. (1978): Review of: "Ever Since Darwin: Reflections on Natural History" by S. J. GOULD, 1977. New York, W. W. Norton, 285 pp. - Systematic Zoology, 27(2): 264-266. Lawrence.
MAYR, E. (1969): Principles of Systematic Zoology, 428 pp., McGraw-Hill. New York.
MIVART, St. G. (1871): On the Genesis of Species. Second Edition. 342 pp., Macmillan, London, New York.
MORGAN, Th. H. (1903): Evolution and Adaptation, 470 pp., Macmillan. New York, London.
NIJHOUT, H. F. (1978): Wing pattern formation in Lepidoptera: a model. - Journal of Experimental Zoology, 206: 119-136. Baltimore.
ODUM, E. P. (1959): Homeostasis of the ecosystem in relation to animal populations, p. 783-784. In: Proceedings, 15th International Congress of Zoology, London, 16-23 July 1958. London.
ODUM, E. P. (1971): Fundamentals of Ecology, 3rd Edition, 574 pp., W. B. Saunders. Philadelphia, London, Toronto.
ODUM, H. T. (1983): Systems Ecology: An Introduction, 644 pp., John Willey & Sons. New York, Chichester, Brisbane, Toronto, Singapore.
OWEN, D. (1980): Camouflage and Mimicry, 158 pp., The University of Chicago Press. Chicago.
PANCHEN, A. L. (1992): Classification, Evolution, and the Nature of Biology. 403 pp., Cambridge University Press. Cambridge, New York, Oakleigh (Victoria).
PARKER, G. H. (1948): Animal Colour Changes and Their Neurohumours: A Survey of Investigations, 1910 to 1943, 377 pp., Cambridge University Press. Cambridge (England).
PIETSCH, Th. W. - GROBECKER, D. B. (1978): The Compleat Angler: Aggressive Mimicry in an Antennariid Anglerfish. - Science, 201(4353): 369-370. Washington, D. C.
PIETSCH, Th. W. - GROBECKER, D. B. (1990): Frogfishes. - Scientific American, 262(6): 96-103. New York.
PLINIUS, Gaius Secundus (1st century A. D.): Naturalis Historia, Engl. Transl. 1887, Natural History, Transl. by J. Bostock and H. T. Riley, 6 vols.
POPPER, K. R. (1974): Darwinism as a metaphysical research program. In: P. A. SCHILLP (Ed.): The Philosophy of Karl Popper, Open Court. La Salle.
POPPER, K. R. (1976): Unended Quest: An Intellectual Autobiography, Revised Edition, 255 pp., Open Court Publish. Co. La Salle (Illinois).
POSTLETHWAIT, J. H. - HOPSON, J. L. (1989): The Nature of Life, 820 pp., McGraw-Hill. New York, Hamburg, London, Paris, Sao Paulo, Sydney, Tokyo, Toronto.
POULTON, E. B. (1890): The colours of animals, their meaning and use, especially considered in the case of insects, 360 pp., The International Scientific Series, vol. 68, Kegan Paul, Trench, Trübner, & Co. Ltd. London.
PROKOP, R. J. - PETR, V. (1986): Revision of the superfamily Melocrinitacea d'Orbigny, 1852 (Crinoidea, Camerata) in Silurian and Devonian of Bohemia. - Sborník Národního muzea, øada B, 42 (3/4): 197-219. Praha.
PROKOP, R.J. - PETR, V. (1987): Marhoumacrinus legrandi, gen. et sp. n. (Crinoidea, Camerata) from Upper Silurian - Lowermost Devonian of Algeria. - Sborník Národního muzea, øada B, 43(1): 1-14. Praha.
PROKOP, R. J. - PETR, V. (1994): A note on the phylogeny of scyphocrinitid crinoids. - Acta Universitatis Carolinae, Geologica, 1992(1-2): 31-36. Praha.
RAYMOND, P. E. (1922): A trilobite retaining color markings. - American Journal of Science, 4: 461-464. New Haven.
ROBINSON, M. H. (1969): Defenses against visually hunting predators, p. 225-259. In: Th. DOBZHANSKY, M. K. HECHT & W. C. STEERE (Eds.): Evolutionary Biology, 3, Appleton Century Crofts. New York.
ROSEN, D. E. (1978): Darwin´s Demon: Review of "Introduction
to Natural Selection". by C.
JOHNSON, 1976. - Systematic Zoology, 27(3): 370-373.
Lawrence.
ROTHSCHILD, M. (1967): Mimicry: The deceptive way of life. - Natural History, 76(2): 44-51. New York.
RUSSEL-HUNTER, W. D. (1968): A Biology of Lower Invertebrates, 181 pp., The Macmillan Company, Collier-Macmillan Limited. London.
SALTHE, S. N. (1993): Development and Evolution: Complexity and Change in Biology, 357 pp., A Bradford Book, The MIT Press. Cambridge (Mass.), London.
SAUNDERS, P. T. (1985): Development and Evolution: Some
Examples, p. 105-115. In: J.
MLÍKOVSKÝ & V. J. A. NOVÁK (Eds.): Evolution and
Morphogenesis, Proceedings of the International Symposium, Plzeò, 1984,
vol. I, Czechoslovak Academy of Sciences. Praha.
SAUNDERS, P. T. - HO, M.-W. (1984): The complexity of organisms, p. 121-139. In: J. W. POLLARD (Ed.): Evolutionary Theory: Paths into the Future. 271 pp., Willey. Chichester, New York, Brisbane, Toronto, Singapore.
SCHMALFUSS, H. (1978a): Structure, Patterns, and Function of Cuticular Terraces in Recent and Fossil Arthropods, I. Decapod Crustaceans. - Zoomorphologie, 90: 19-40. Berlin, Heidelberg, New York.
SCHMALFUSS, H. (1978b): Constructional morphology of cuticular terraces in crustaceans. - Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 157: 155-159. Stuttgart.
SCHMALFUSS, H. (1978c): Constructional morphology of cuticular terraces in trilobites, with conclusions on synecological evolution. - Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen, 157: 164-168. Stuttgart.
SCHMALFUSS, H. (1981): Structure, patterns and function of cuticular terraces in trilobites. - Lethaia, 14(4): 331-341. Oslo.
SCHMIDT-KITTLER, N. - VOGEL, K. (Eds.) (1991): Constructional Morphology and Evolution, 409 pp., Springer-Verlag. Berlin, Heidelberg, London, Paris, Tokyo, Hong Kong, Barcelona, Budapest.
SEPKOSKI, J. J., Jr. (1981): A factor analytic description of the Phanerozoic marine fossil record. - Paleobiology, 7: 36-53. Ithaca.
SEPKOSKI, J. J., Jr. (1991): A model of onshore-offshore change in faunal diversity. - Paleobiology, 17: 58-77. Ithaca.
SEPKOSKI, J. J., Jr. (1995): The Ordovician radiations: Diversification and extinction shown by global genus-level taxonomic data, p.393-396. In: J. D. COOPER, M. R. DROSER & S. C. FINNEY (Eds.): Ordovician Odyssey: Short Papers for the Seventh International Symposium on the Ordovician System, Las Vegas, Nevada, USA, June 1995, 498 pp., The Pacific Section Society for Sedimentary Geology (SEPM). Fullerton (California).
SEPKOSKI, J. J., Jr. - MILLER, A. I. (1985): Evolutionary faunas and the distribution of Paleozoic benthic communities in space and time, p. 153-190. In: J. W. VALENTINE (Ed.): Phanerozoic Diversity Patterns: Profiles in Macroevolution, Princeton University Press and Pacific Division, American Association for the Advancement of Science. Princeton (New Jersey).
SEPKOSKI, J. J., Jr. - SHEEHAN, P. M. (1983): Diversification, faunal change, and community replacement during the Ordovician radiations, p. 673-717. In: M. J. S. TEVESZ & P. M. McCALL (Eds.): Biotic Interactions in Recent and Fossil Benthic Communities, Plenum Press. New York.
SHAW, G. B. (1921): Back to Methuselah. A Metabiological Pentateuch,. (see only the author´s foreword), Brentano´s. New York.
SHEAR, W. A. - KUKALOVÁ-PECK, J. (1990): The ecology of Paleozoic terrestrial arthropods: the fossil evidence. - Canadian Journal of Zoology, 68: 1807-1834. Ottawa.
SILBERGLIED, R. E. - AIELLO, A. (1980): Camouflage by Integumentary Wetting in Bark Bugs. - Science, 207(4432): 773-775. Washington, D. C.
SIMPSON, G. G. (1944): Tempo and mode in evolution. 237 pp., Columbia Biological Series 15, Columbia Univ. Press. New York.
SIMPSON, G. G. (1949): The Meaning of Evolution: A Study of the History of Life and of its Significance for Man. 364 pp., Yale Univ. Press. New Haven, London.
SIMPSON, G. G. (1953): The Major Features of Evolution, (4th printing 1965), 434 pp., Columbia University Press. New York.
STEPHENSON, E. M. (1946): Animal Camouflage. Pelican Books. Harmsworth, Middlesex.
SULLIVAN, R. M. - LUCAS, S. G. - HUNT, A. P. - FRITTS, Th. H. (1988): Color pattern on the selmacryptodiran turtle Neurankylus from the early Paleocene (Puercan) of the San Juan Basin, New Mexico. - Contributions in Science, 401: 1-9. Natural History Museum of Los Angeles County.
SWYNNERTON, C. F. M. (1926): An investigation into the defences of butterflies of the genus Charaxes, p. 478-506. In: Proceedings of the 3rd International Entomological Congress, Zurich 1925, Vol. 2.
TEICHERT, C. (1944): Permian trilobites from Western Australia. - Journal of Paleontology, 18: 445-465. Menasha.
THOMAS, L. (1971): Sensuous Symbionts of the Sea. - Natural History, 80(7): 28-37. New York.
THULBORN, T. (1994): Mimicry in ankylosaurid dinosaurs. - Records of the South Australian Museum, 27(2): 151-158. Adelaide.
TRESHER, R. E. (1978): The Ornamental Eye. - Natural History, 87(2): 84-89. New York.
VANE-WRIGHT, R. J. (1976): A unified classification of mimetic resemblances. - Biological Journal of the Linnean Society, 8: 25-56. London.
VERMEIJ, G.J. (1982): Unsuccessful predation and evolution. - The American Naturalist, 120: 701-720.
VERNADSKIJ, V. I. see VERNADSKY, W. I.
VERNADSKY, W. I. (1929): La Biosphere, 232 pp., Librairie Félix Alcan. Paris.
VERNADSKY, W. I. (1945): The biosphere and the noosphere. - American Scientist, 33: 1-12.
WALLACE, A. R. (1889): Darwinism: An Exposition of the Theory of Natural Selection with Some of Its Applications, 494 pp., Macmillan & Co. London, New York.
WALLACE, A. R. (1895): Natural Selection and Tropical Nature: Essays on Descriptive and Theoretical Biology. New Edition with Corrections and Additions. 492 pp., Macmillan. London, New York.
WARD, P. D. (1982): Nautilus: Have Shell, Will Float. - Natural History, 91(10): 65-69. New York.
WATTS, A. (1970): The World Is Your Body (reprinted from the author´s: The Book: On the Taboo Against Knowing Who You Are, Pantheon Books 1966), p. 181-193. In: DISCH, R. (Ed.): The Ecological Conscience: Values for Survival, 206 pp., Prentice-Hall Inc. Englewood Cliffs (New Jersey).
WEBB, G. E. (1994): Parallelism, non-biotic data and phylogeny reconstruction in paleobiology. - Lethaia, 27(3): 185-192. Oslo.
WEBBY, B. D. (1992): Ordovician island biotas: New South Wales record and global implications. - Journal and Proceedings, Royal Society of New South Wales, 125(3/4): 51-77. Macquarie Centre.
WELLS, J. W. (1942): Supposed color-markings in Ordovician trilobites from Ohio. - American Journal of Science, 240(10): 710-713. New Haven.
WELLS, M. J. (1962): Brain and Behaviour in Cephalopods, 171 pp., University Biology Monographs, Stanford University Press. Stanford (California).
WESTROP, S. R. (1983): The life habits of the Ordovician illaenine trilobite. - Lethaia, 16(1): 15-24. Oslo.
WESTROP, S. R - TREMBLAY, J. V. - LANDING, E. (1995): Declining Importance of Trilobites in Ordovician Nearshore Paleocommunities: Dilution or Displacement?. - Palaios, 10(1): 75-79. SEPM, Tulsa, and Allen Press, Lawrence.
WHITE, C. S. - MICHAUX, B. - LAMBERT, D. M. (1990): Species and neo-Darwinism. - Systematic Zoology, 39(4): 399-413. Washington, D. C.
WHITTINGTON, H. B. (1959): Ontogeny of Trilobita, O127-O145. In: R. C. MOORE (Ed.): Treatise on Invertebrate Paleontology, Part O, Arthropoda 1. Geological Society of America, Boulder, and University of Kansas Press, Lawrence (Kansas).
WHITTINGTON, H. B. (1966): Phylogeny and distribution of Ordovician trilobites. - Journal of Paleontology, 40: 696-737. Menasha.
WICKLER, W. (1968): Mimicry in Plants and Animals, 255 pp., World University Library, McGraw-Hill. New York.
WILLIAMS, G. C. (1966): Adaptation and Natural Selection: A Critique of Some Current Evolutionary Thought, 307 pp., Princeton University Press. Princeton (New Jersey).
WILLIAMS, J. S. (1930): A color pattern on a new Mississippian trilobite. - American Journal of Science, 20(115): 61-64. New Haven.
WILSON, D. S. (1988): Holism and reductionism in evolutionary
ecology. - Oikos, 53(2): 269-273. Copenhagen.
Explanations to the text-figures:
Text-fig. 1: Two examples of accidental homeomorphy or (Batesian) mimicry? Coexisting Ordovician trilobite genera from the Barrandian area: 1. Prionocheilus ROUAULT (upper left) and Flexicalymene SHIRLEY (upper right), 2. Deanaspis HUGHES, INGHAM et ADDISON (lower left) and Dionide GUERICH (lower right).
 a) a) |
 b) b) |
Pl. I.: Boeckops boecki (HAWLE et CORDA)
Fig, 1: medium-grained sculpture on the cephalon of an
enrolled individual from Damil (Chlupáè 1977, Tab. XV, fig. 11). x2.2.
Fig. 2: almost smooth cephalon (Chlupáè 1977, Tab. XV,
fig. 3) x2.6.
Fig. 3: coarse granulation on the cephalon of an enrolled
specimen from Damil (Chlupáè 1977, Tab. XV, fig. 12) x2.2.
Pl. II.: Reedops bronni (BARRANDE)
Fig. 1: coarse granulation on the cephalon from Praha
- Konváøka (Chlupáè 1977, Tab. XXII, fig. 16) x3.6.
Fig. 2: left lateral view of dtto (Chlupáè 1977, Tab.
XXII, fig. 17) x3.6.
Fig. 3: sparsely granulated to almost smooth cephalon
from Branovy near Lodìnice (Chlupáè 1977, Tab. XXII, fig. 18) x2.6.
|
|
|
|
|
|
|